Method for preparing zinc blende nano particle and zinc blende nano particle prepared thereby
A technology of nano-particles and zinc sulfide, applied in the direction of zinc sulfide, nanostructure manufacturing, nanotechnology, etc., can solve the problems of easy oxidation of sulfide, unreachable samples, and easy occurrence of danger, etc., and achieve low reaction temperature and no pollution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] (1) Preparation of ZnS precursor:
[0023] Select the microemulsion system of CTAB / n-butanol / cyclohexane / water, cyclohexane is the oil phase, CTAB (cetyltrimethylammonium bromide) is the surfactant, and n-butanol is the co-surfactant agent, Zn(NO 3 ) 2 ·6H 2 O solution and Na 2 S·9H 2 The O solution is the aqueous phase. Two microemulsions, I and II, need to be configured during the test. The aqueous phase in microemulsion I was 0.5M Zn(NO 3 ) 2 ·6H 2 O solution, the aqueous phase in microemulsion II is 0.5M Na 2 S solution. 2g CTAB, 20ml cyclohexane and 4ml n-butanol were mixed and dissolved. Under constant stirring, slowly add 2.4ml of 0.5M Zn(NO 3 ) 2 ·6H 2 O solution, to ensure that the system is in a transparent state without delamination, to obtain microemulsion I. Microemulsion II differs in that 0.5M Na 2 S solution replaced 0.5M Zn(NO 3 ) 2 ·6H 2 O solution. The two transparent and stable microemulsions I and II were quickly mixed and stirre...
Embodiment 1
[0026] Example 1 : Preparation of ZnS powder
[0027] Preparation of ZnS precursor: Mix 2g CTAB, 20ml cyclohexane and 4ml n-butanol to dissolve CTAB to form an oil phase solution. Slowly add 2.4ml of 0.5M Zn(NO 3 ) 2 ·6H 2 O solution to obtain microemulsion I. Similarly, slowly add 2.4ml of 0.5M Na to the oil phase solution 2S solution to obtain microemulsion II. The microemulsion I and microemulsion II were rapidly mixed and stirred continuously for 30 minutes to obtain an emulsion containing a white precipitate. Let it stand at room temperature for 12h, then add the demulsifier acetone and separate the precipitate with a centrifuge. The precipitate was washed 5 times with acetone, 3 times with ethanol, and 3 times with deionized water, and then dried at room temperature to obtain the ZnS precursor.
[0028] Hydrothermal reaction: move the prepared ZnS precursor into a 100ml polytetrafluoroethylene-lined autoclave, add distilled water to 70% of the volume of the auto...
Embodiment 2
[0031] Example 2 : Preparation of ZnS powder
[0032] The ZnS precursor was prepared by the same method as in Example 1.
[0033] Hydrothermal reaction: move the prepared ZnS precursor into a 100ml polytetrafluoroethylene-lined autoclave, add distilled water to 70% of the volume of the autoclave, seal it, and react at a hydrothermal temperature of 160°C for 12 hours. After washing with distilled water for 3 times and absolute ethanol for 3 times, dry in a vacuum oven at 60°C for 6 hours to obtain a powder which is the product.
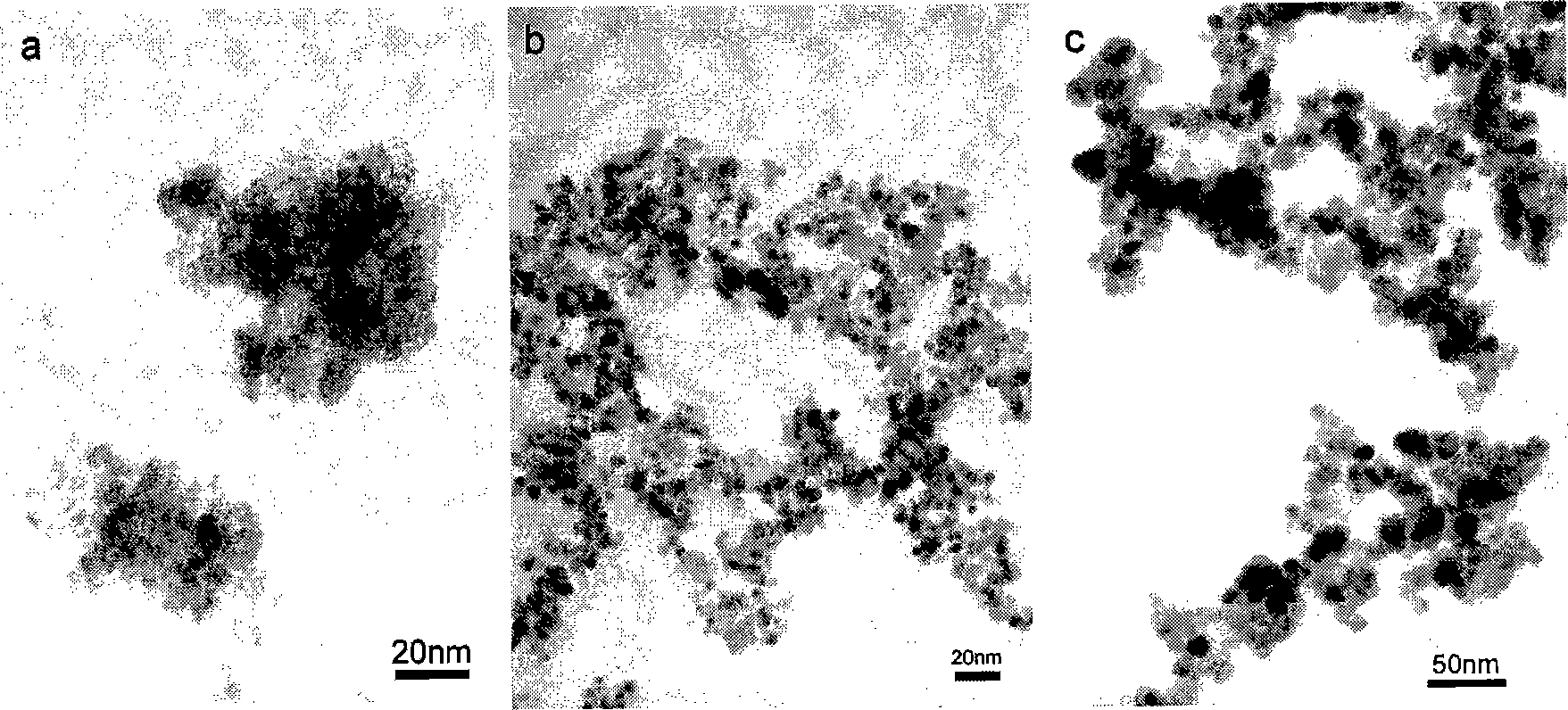
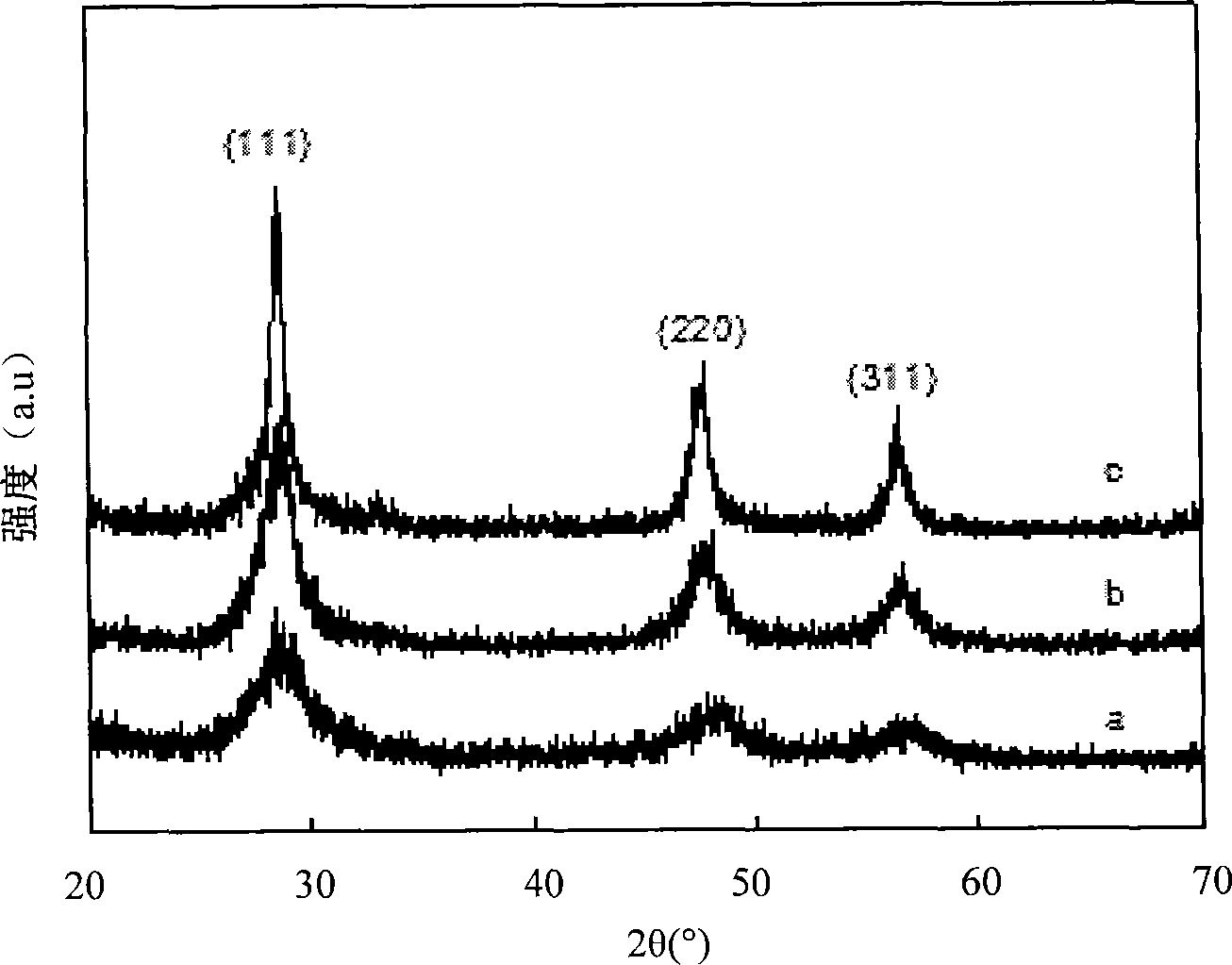
[0034] Identification: The product is identified as cubic phase ZnS by X-ray diffraction (XRD), such as image 3 (b) shown. The transmission electron microscope observation shows that the product is composed of uniformly dispersed spherical particles with an average size of 5 nm, such as figure 1 (b) shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average grain size | aaaaa | aaaaa |
| Average size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com