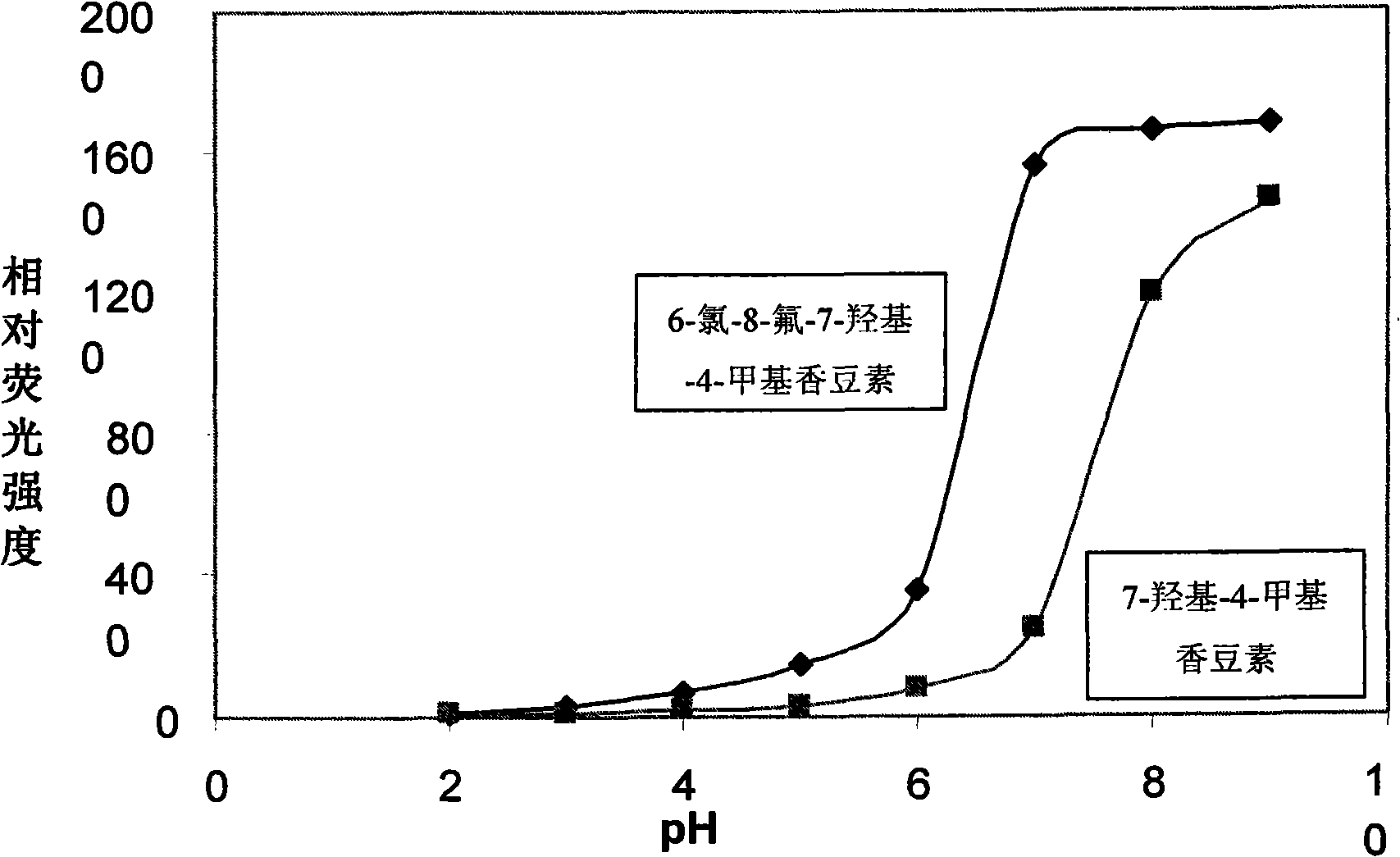
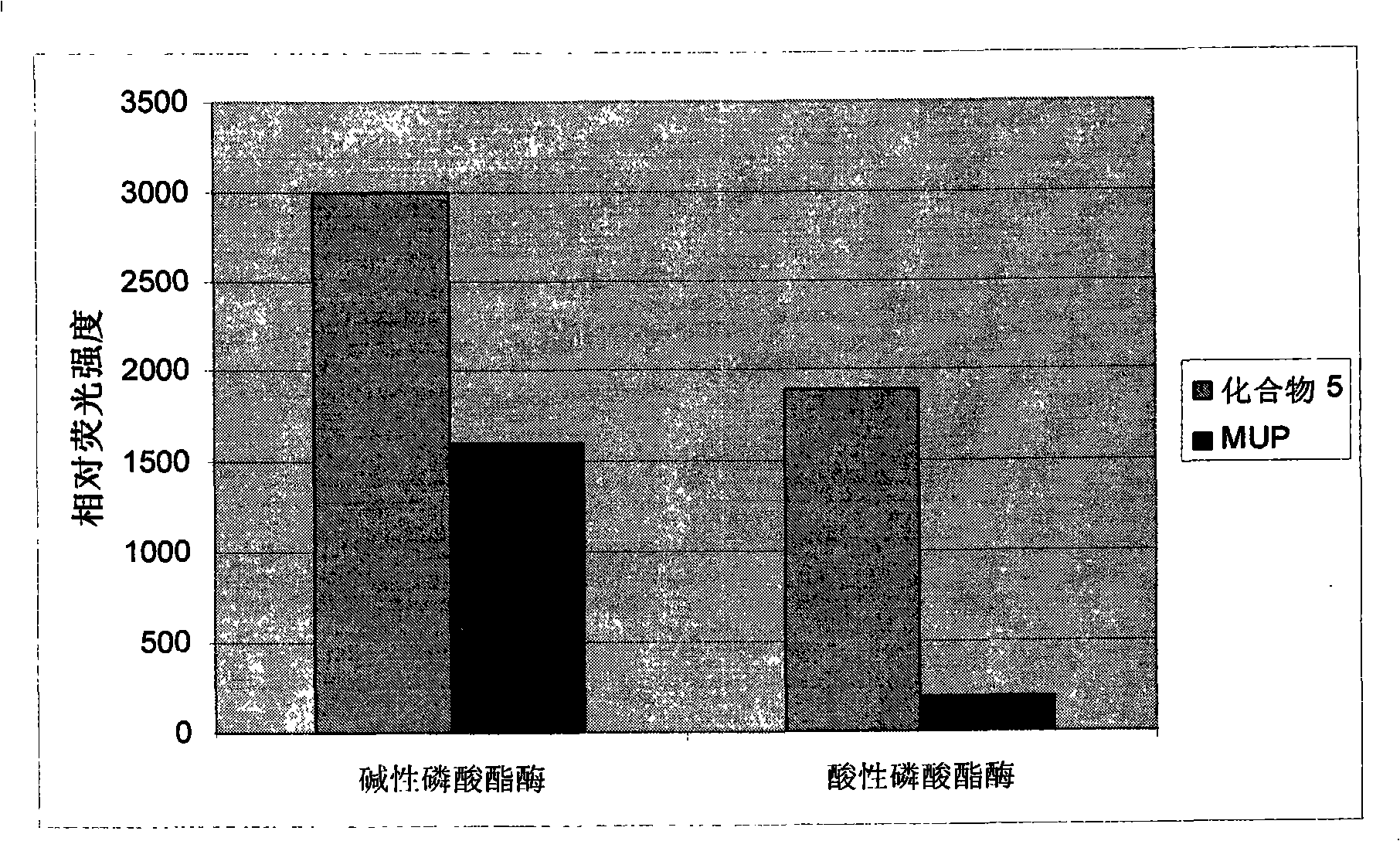
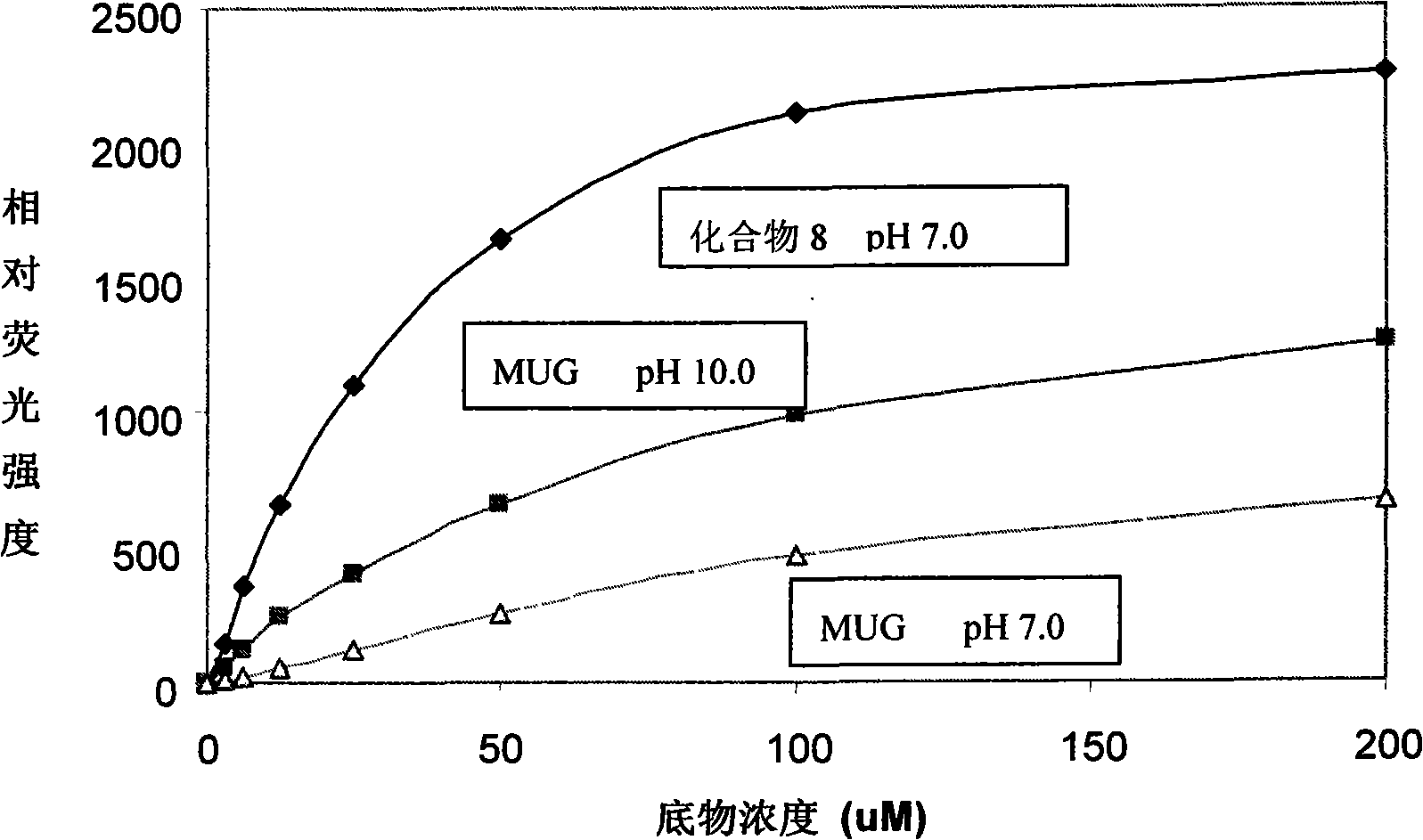
Coumarin compound for enzymatic activity analysis and enzyme inhibitor sifting motion, and synthesizing process
A technology for inhibitor screening and coumarins, which is applied in the fields of compounds of Group 5/15 elements of the periodic table, chemical instruments and methods, material excitation analysis, etc. There are few types of bean-based fluorescent dyes, low sensitivity and accuracy, etc., to achieve good solubility, high photostability, and improved sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 of the present invention: Synthesis of 6-chloro-8-fluoro-7-hydroxyl-4-methylcoumarin (compound 2)
[0024] ①Synthesis of 5-chloro-3-fluoro-resorcinol (compound 1).
[0025]
[0026]Add 100g (0.565mole) 2,3,4-trifluoronitrobenzene and 1000ml of anhydrous methanol into a 2000ml three-path bottle, cool to below 0~-5°C with an ice-salt bath, and stir electromagnetically. 335.6 g (1.243 mole) of 20% sodium methoxide in methanol was added dropwise within 2-2.5 h. Stir at room temperature for 10h. The progress of the reaction was detected by TLC, and the pH=7 was neutralized with 1 mole / L citric acid after the reaction. The above reaction system was dispersed in 2000 ml of water, and the precipitated light yellow solid was filtered, washed with water and dried. Recrystallized from absolute ethanol to obtain light yellow crystals of 2-fluoro-4-nitro-1,3-benzenediol dimethyl ether.
[0027] Into a 1000 ml round bottom flask, add 20 g of 2-fluoro-4-nitro-1,3-benz...
Embodiment 2
[0032] Example 2 of the present invention: Synthesis of 6-chloro-8 fluoro-7-hydroxyl-3-carboxycoumarin (compound 4)
[0033] 1. Synthesis of 5-chloro-3-fluoro-2,4-dihydroxybenzaldehyde (compound 3):
[0034]
[0035] 10 g of 2-fluoro-4-chloro-1,3-benzenediol, 117.2 g of hexamethylenetetramine, and 80 ml of trifluoroacetic acid were sequentially added to a 250 ml three-necked flask. Electromagnetic stirring was carried out until it was completely dissolved, and the temperature was raised to 90°C for 24 hours. After the reaction was complete, cool to room temperature, add 100 ml of 20% H 2 SO 4 , after stirring for 1 h, the reaction mixture was poured into water and extracted with ethyl acetate (500 mL×2). The organic phase was washed with saturated brine, dried over anhydrous MgSO4, concentrated, washed with ethyl acetate / n-hexane and crystallized to obtain white crystals of 3-fluoro-5-chloro-2,4-dihydroxybenzaldehyde.
[0036] ② Synthesis of 6-chloro-8-fluoro-7-hydroxy-...
Embodiment 3
[0040] Example 3 of the present invention: Synthesis of phosphatase substrate-6-chloro-8-fluoro-7-hydroxyl-4-methylcoumarin phosphate (compound 5)
[0041]
[0042] at 0°C, N 2 Under protection, the pyrimidine solution containing compound 2 was added to the POCl solution 3 (2 molar) pyrimidine solution, the reaction was followed by TLC. After the reaction, the solution was poured into a beaker filled with crushed ice, and the pH of the solution was adjusted to 5 with ammonia water. Then, pyrimidine was extracted with chloroform, and the aqueous phase was partially lyophilized to obtain a crude product.
[0043] The product was purified by Sephadex gel chromatography using a SEPHADEX LH 20 gel column with water as the eluent. The pure product was collected and frozen to obtain colorless crystals. The solid was dissolved in water, and DOWEX 50.4-200 strongly acidic cation exchange resin was added under stirring, then the resin was removed by filtration, the resin was wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com