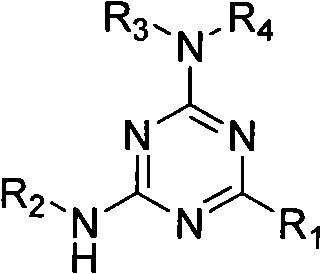
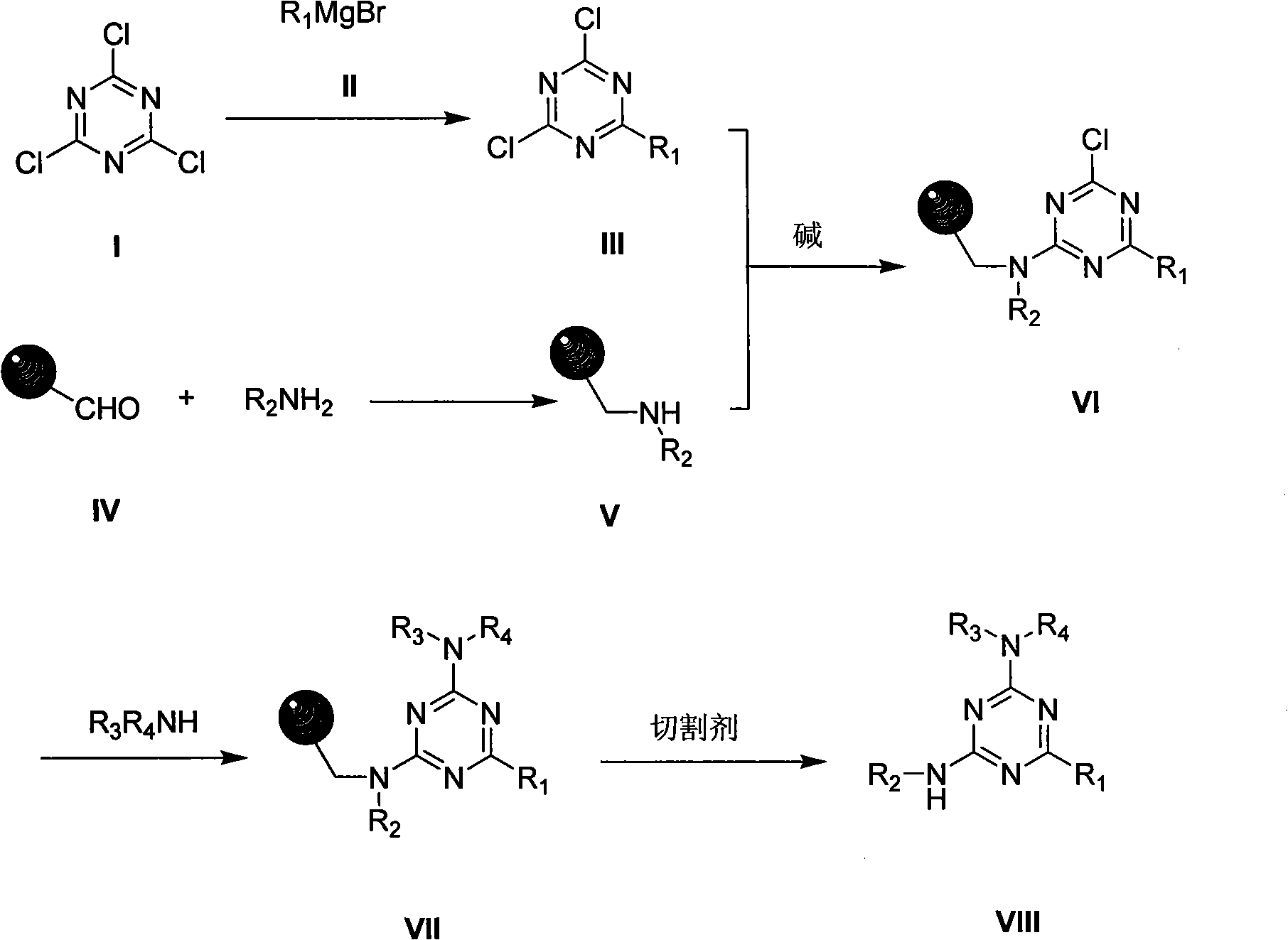
2,4,6-tri-substituted-1,3,5-triazine derivates library and preparation method
A technology of trisubstitution and triazines, applied in 2 fields, to achieve the effects of high purity and yield, mild synthesis conditions, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Preparation of 2,4-dichloro-6-phenyl-1,3,5-triazine III
[0026] Add 0.78g (33mmol) magnesium bars and 20mL anhydrous tetrahydrofuran (THF) into a 50mL three-necked flask, add a few grains of iodine to activate, slowly add 4.7g (30mmol) bromobenzene to the system, and keep the system in a slightly boiling state. After the dropwise addition was completed, keep the temperature at 65°C for 2 hours. After cooling the room temperature, slowly add the Grignard reagent dropwise to the THF (30mL) solution of cyanuric chloride (2.8g, 15mmol) cooled to 0°C in advance, and keep the internal temperature 0~10°C, react at 20°C for 3 hours after the dropwise addition, dilute the mixture with toluene, wash with 10% hydrochloric acid, wash with water, distill off the solvent under reduced pressure to obtain a yellow solid 2,4-dichloro-6-phenyl-1 , crude product of 3,5-triazine III. Yield 66%, m.p. 110°C.
Embodiment 2
[0027] Example 2: Preparation of 2,4-dichloro-6-(4-methoxyphenyl)-1,3,5-triazine III
[0028]Add 4.2g (176mmol) magnesium bars and 60mL anhydrous THF into a 100mL three-necked flask, add a few grains of iodine to activate, slowly add 24.3g (130mmol) p-bromoanisole into the system, and keep the system in a slightly boiling state. After the dropwise addition was completed, it was kept at 65°C for 0.5 hours. After cooling to room temperature, the Grignard reagent was slowly added dropwise to a THF (60mL) solution of cyanuric chloride (12g, 65mmol) cooled to 0°C in advance, and the internal temperature was kept at 0°C. ~10°C, react at 20°C for 3 hours after the dropwise addition, dilute the mixture with toluene, wash with 10% hydrochloric acid, wash with water, distill off the solvent under reduced pressure to obtain yellow-white solid 2,4-dichloro-6-(4-methyl Crude product of (oxyphenyl)-1,3,5-triazine III. Yield 61%, m.p. 122-124°C.
Embodiment 3
[0029] Example 3: Preparation of 2,4-dichloro-6-(3-chlorophenyl)-1,3,5-triazine III
[0030] Add 3.0g (125mmol) magnesium bars and 50mL anhydrous THF into a 100mL three-necked flask, add a few grains of iodine to activate, slowly add 22.8g (119mmol) m-chlorobromobenzene to the system, and keep the system in a slightly boiling state. After the dropwise addition was completed, keep the temperature at 65°C for 2 hours. After cooling the room temperature, slowly add the Grignard reagent dropwise to the THF (60mL) solution of cyanuric chloride (11.1g, 60mmol) cooled to 0°C in advance, and keep the internal temperature 0~10°C, react at 20°C for 3 hours after the dropwise addition, dilute the mixture with toluene, wash with 10% hydrochloric acid, wash with water, distill off the solvent under reduced pressure to obtain yellow solid 2,4-dichloro-6-(3-chloro Crude phenyl)-1,3,5-triazine III. Yield 58%, m.p. 92-95°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com