CXCR4 antagonist recombination protein SDF-1 beta P2G, preparation method and application thereof
A recombinant protein, SDF-1 technology, applied in the biological field, can solve problems such as inability to continuously promote blood flow recovery, achieve the effects of inhibiting growth and metastasis, restoring function, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
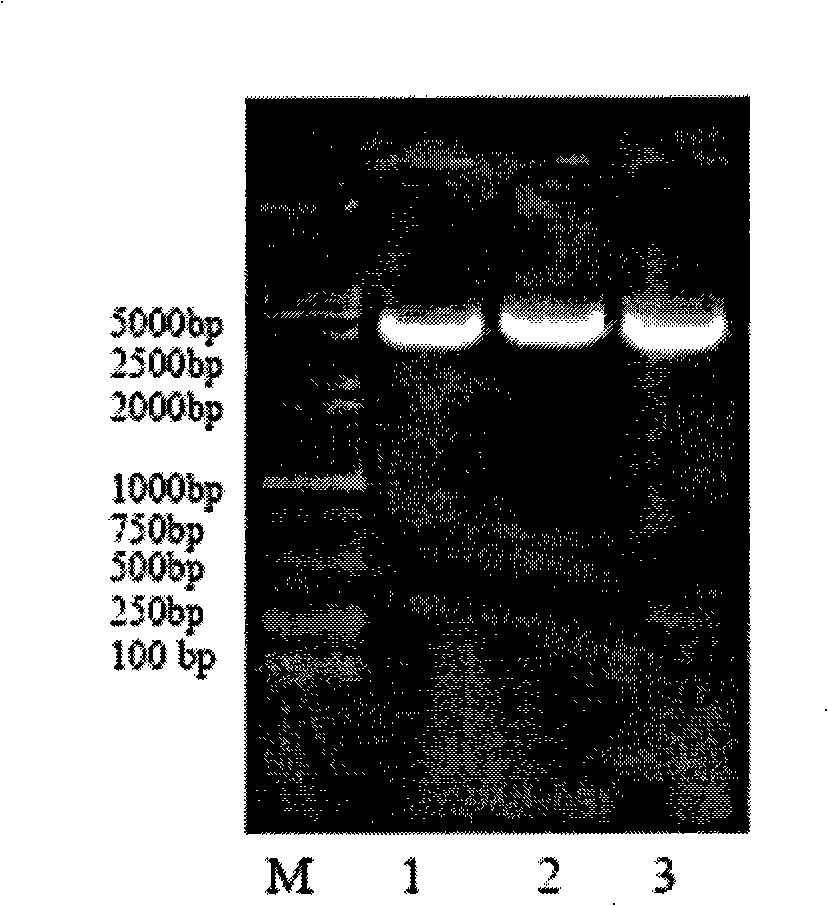
[0053] Cloning of SDF-1βP2G cDNA and Construction of Prokaryotic Expression System
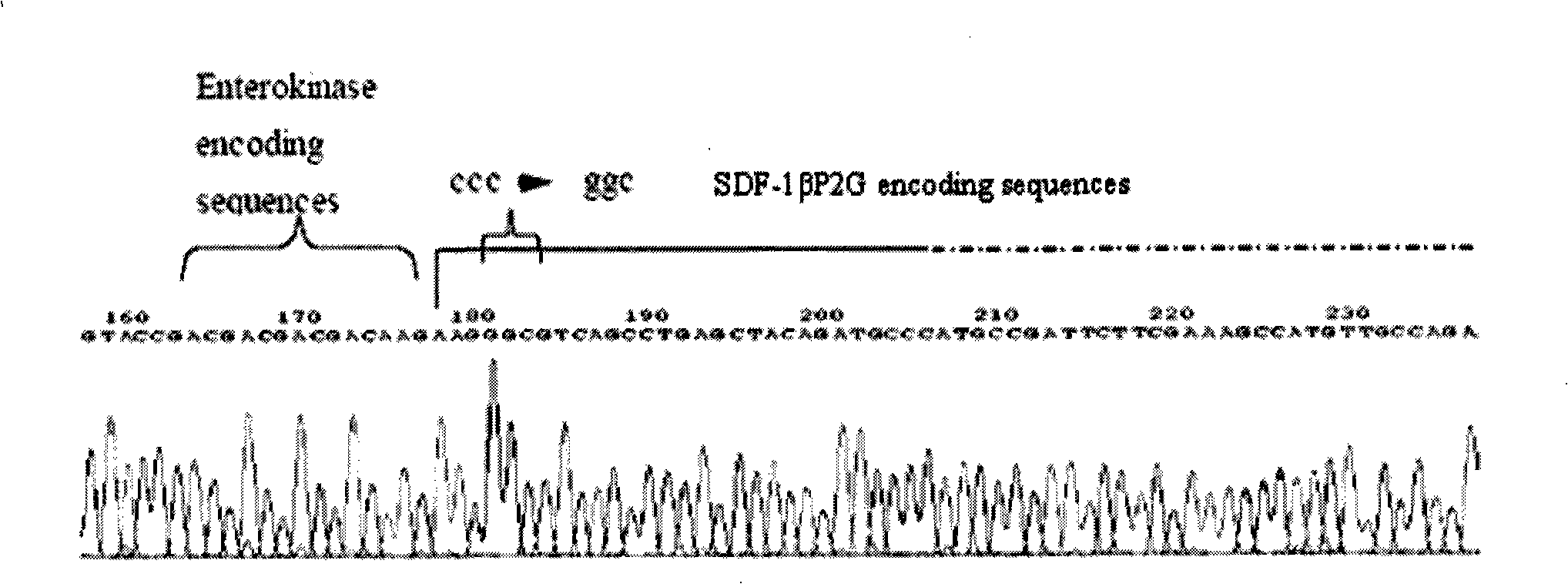
[0054] 1. Primer design: According to the cDNA sequence of human SDF-1β provided by the Genebank database, according to the general primer design principles and the requirements of PET30a(+) cloning, use primer5.0 to design RT-PCR primer SDF-1βP2G-F with the coding region as a template / SDF-1βP2G-R, directly amplifies the mutant SDF-1βP2G in which the second amino acid residue at the N-terminus of SDF-1β is mutated from proline to glycine.
[0055] Primer SDF-1βP2G-F is: 5'-ggGGTACCgacgacgacgacaagaagggcgtcagcctgagctacagat-3';
[0056] The primer SDF-1β-R is: 5'-acgGAGCTCacatcttgaacctcttgtt-3'.
[0057] 2. Extraction of total RNA: Take out about 0.25ml of bone marrow sample from liquid nitrogen, immediately add 0.75ml TRIZOL LSReagent, after the sample is thawed, blow and beat the bone marrow cells repeatedly with a pipette. The lysed cell homogenate was incubated at 15-30°C for 5 minutes to ...
Embodiment 2
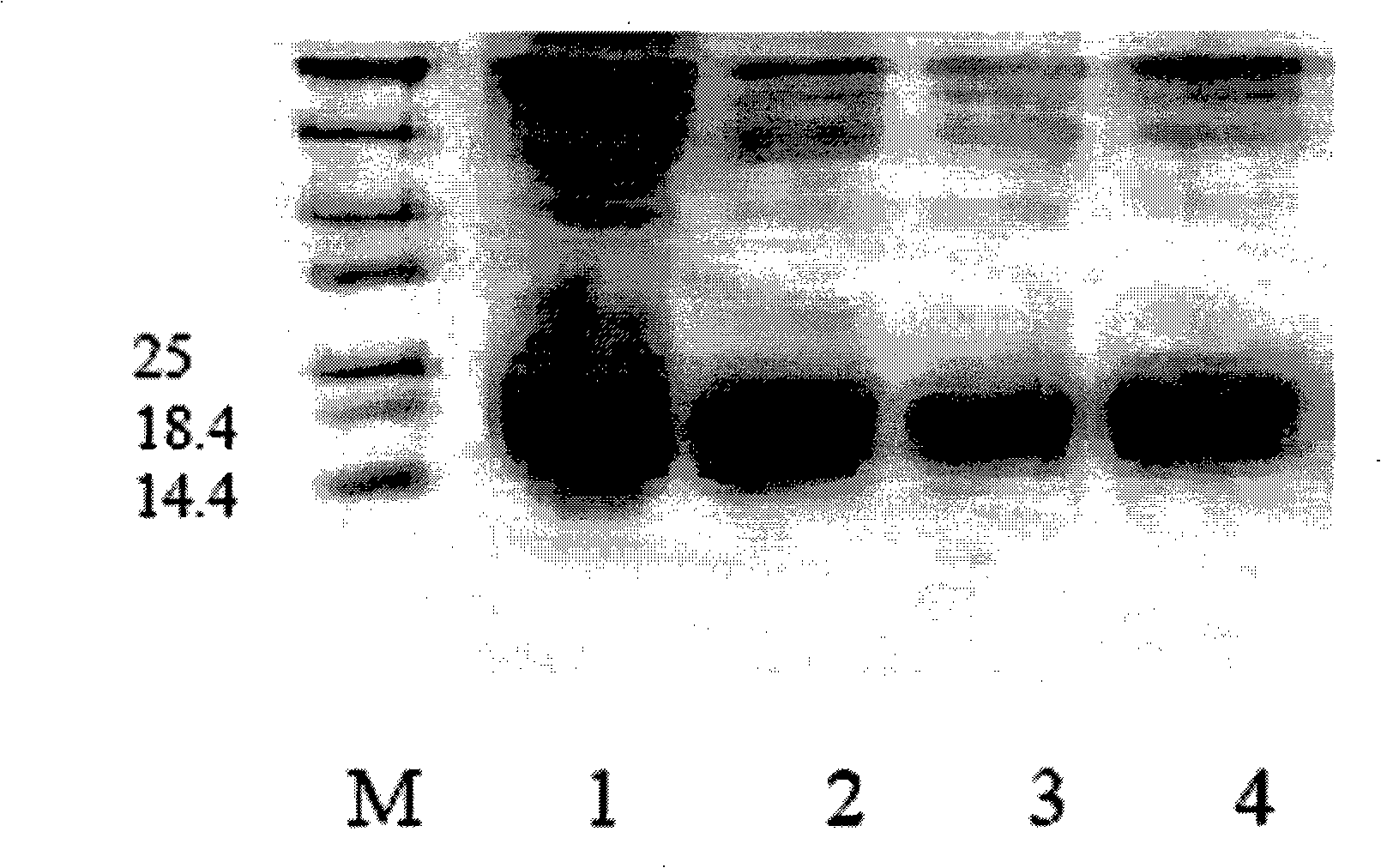
[0085] Prokaryotic expression and purification of recombinant protein SDF-1βP2G
[0086] Add about 200ng of the confirmed SDF-1βP2G / pET-30a(+) recombinant plasmid into 200μl competent Escherichia coli BL21(DE3)(NOVAGEN) cells (prepared by calcium chloride method), mix gently, and place on ice for 30min , then heat-shocked at 42°C for 90s, immediately transferred to ice and placed on ice for 2min, then added 800μl LB culture solution and cultured with shaking at 37°C for 45min, took 2001 and spread it on LB plates containing 50μg / ml kanamycin, at 37°C Cultivate overnight (about 12hr), randomly pick 5 positive colonies and inoculate them in 50ml LB liquid medium (containing 50μg / ml kanamycin) for about 12hr at 37°C, then transfer 50μl of the above culture solution to 50ml of LB liquid medium (containing 50μg / ml kanamycin) was shaken at 37°C for about 3hrs, when OD600=0.5, IPTG with a final concentration of 0.75mM was added to induce expression at 37°C for 3hrs, centrifuged at 30...
Embodiment 3
[0103] SDF-1βP2G antagonizes the activity of CXCR4
[0104] 1. Chemotactic activity
[0105] Take a 24-well plate, add predetermined final concentrations (0, 0.001, 0.01, 0.1, 0.5, 1, 10, 100nmol / L equal concentration gradient) of RPMI1640 dilution of SDF-1β, SDF-1βP2G, and then gently Use tweezers to place the Chemotaxic chamber with pore size of 5μm at the bottom, pay attention to avoid air bubbles, then add 1×10 7Cells / ml MOLT-4 cell suspension 200μL; Incubate in a 5% CO2 incubator at 37°C for 2-3hrs, then gently remove the chemotaxis chamber at one time, and count the number of cells in each well of the cell culture plate under a microscope . Relative Chemotaxic Activity = number of transmembrane cells / total number of uploaded cells × 100%. Each treatment was repeated three times, and the data were processed with DPSv5.02. The results were expressed as Mean±S, and the multiple comparisons were performed by the new multiple range method of DUNCAN.
[0106] The results s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com