Acryloyl di-quaternary ammonium salt and preparation thereof
A technology of acryloyl bisquaternary ammonium salt and purification method, which is applied in the field of quaternary ammonium salt and its preparation, and can solve the problems of lack of long-term antibacterial, poor substrate affinity, low molecular weight, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
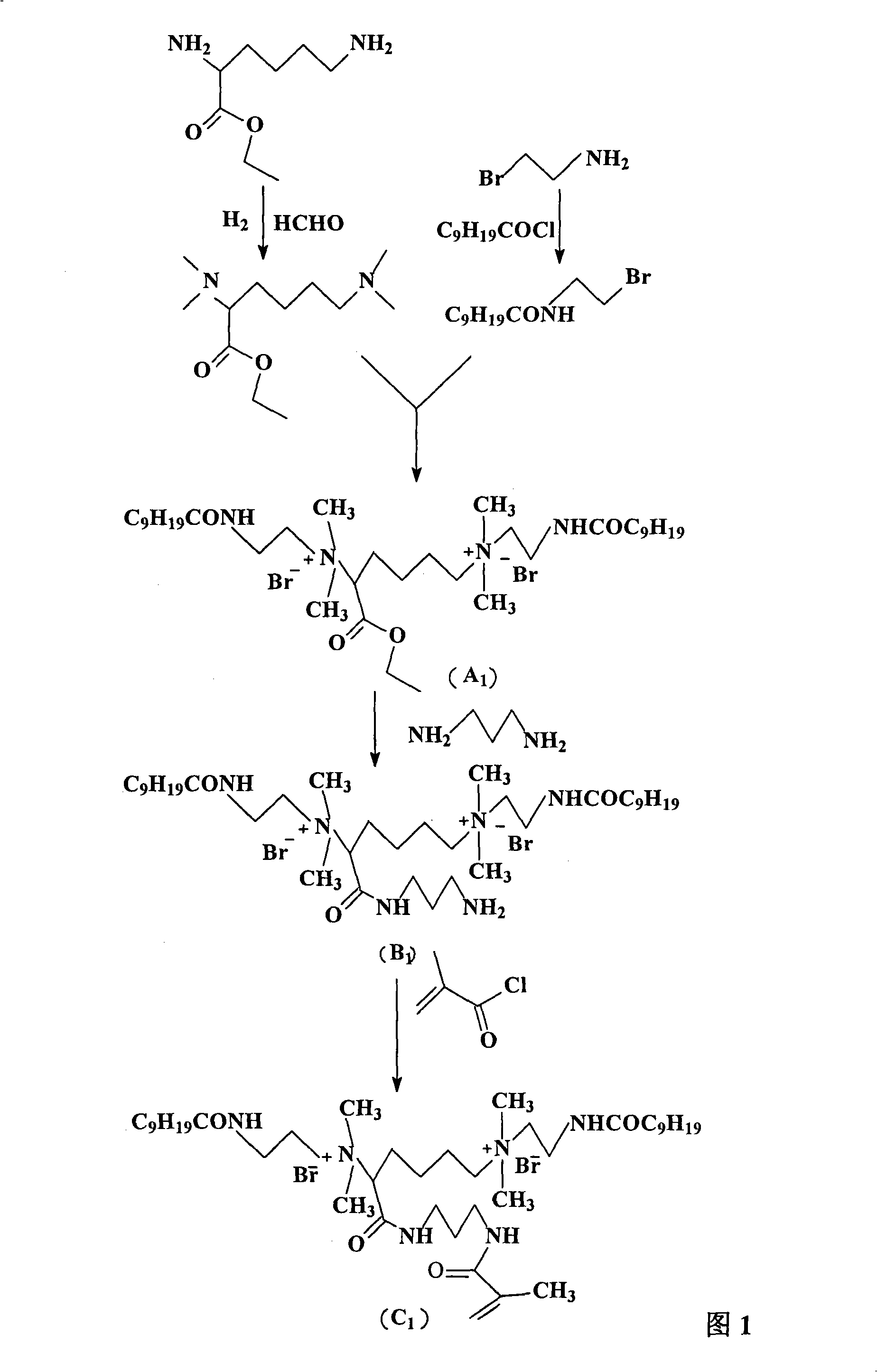
[0050] In this example, acryloyl bis-quaternary ammonium salt (C1) was prepared.
[0051] (1) N, N, N', the preparation of N'-tetramethyl-2,6-diaminocaproic acid ethyl ester
[0052] Dissolve 24.7 grams (0.1mol) of ethyl 2,6-diaminocaproate dihydrochloride in 45 grams of 40% formaldehyde solution, then add 2.47 grams of palladium carbon, hydrogenolyze at room temperature for 12 hours, and filter Remove palladium carbon, then add 16.8 grams of sodium bicarbonate to the filtrate, remove formaldehyde by distillation under reduced pressure, extract the product with ethyl acetate and wash once with saturated saline and deionized water, dry over anhydrous sodium sulfate, and remove ethyl acetate by distillation under reduced pressure ester, namely to obtain 20.7 g of N, N, N', N'-tetramethyl-2,6-diaminocaproic acid ethyl ester with a yield of 90%.
[0053] ( 1 HNMR: 600MHz, CDCl 3 )δppm: 1.32 (3H, t, CH 3 ); 1.47~1.59 (2H, m, CH 2 ); 1.82~2.01 (4H, m, 2CH 2 ); 2.56(6H, s, 2CH ...
Embodiment 2
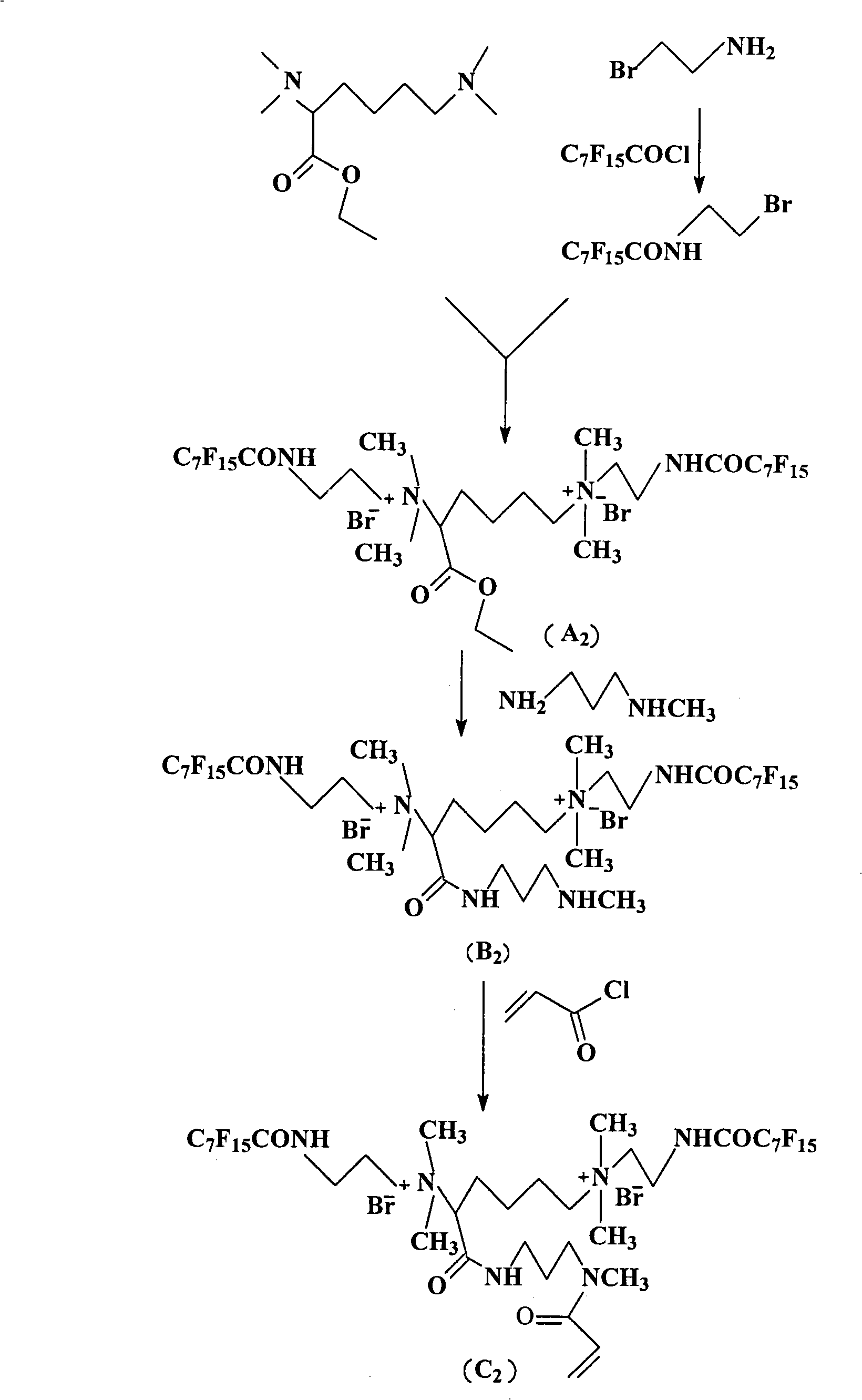
[0071] In this example, acryloyl bis-quaternary ammonium salt (C2) was prepared.
[0072] (1) N, N, N', the preparation of N'-tetramethyl-2,6-diaminocaproic acid ethyl ester
[0073] Because the preparation of N, N, N', N'-tetramethyl-2,6-diaminocaproic acid ethyl ester in this embodiment is exactly the same as that in Example 1, it is omitted here.
[0074] (2) Preparation of perfluorooctanoyl-2-bromoethylamine
[0075] Dissolve 41.0 grams (0.2mol) of 2-bromoethylamine hydrobromide in 250ml of deionized water, then add 33.6 grams (0.4mol) of sodium bicarbonate, lower the temperature to 0°C, and dissolve the solution in 200ml of anhydrous dichloro Add 86.6 g (0.2 mol) of methane perfluorooctanoyl chloride dropwise into 2-bromoethylamine aqueous solution, and react for 2 hours after the dropwise addition; solution, saturated sodium chloride, and deionized water, dried over anhydrous sodium sulfate, filtered, and dichloromethane was distilled off under reduced pressure to obta...
Embodiment 3
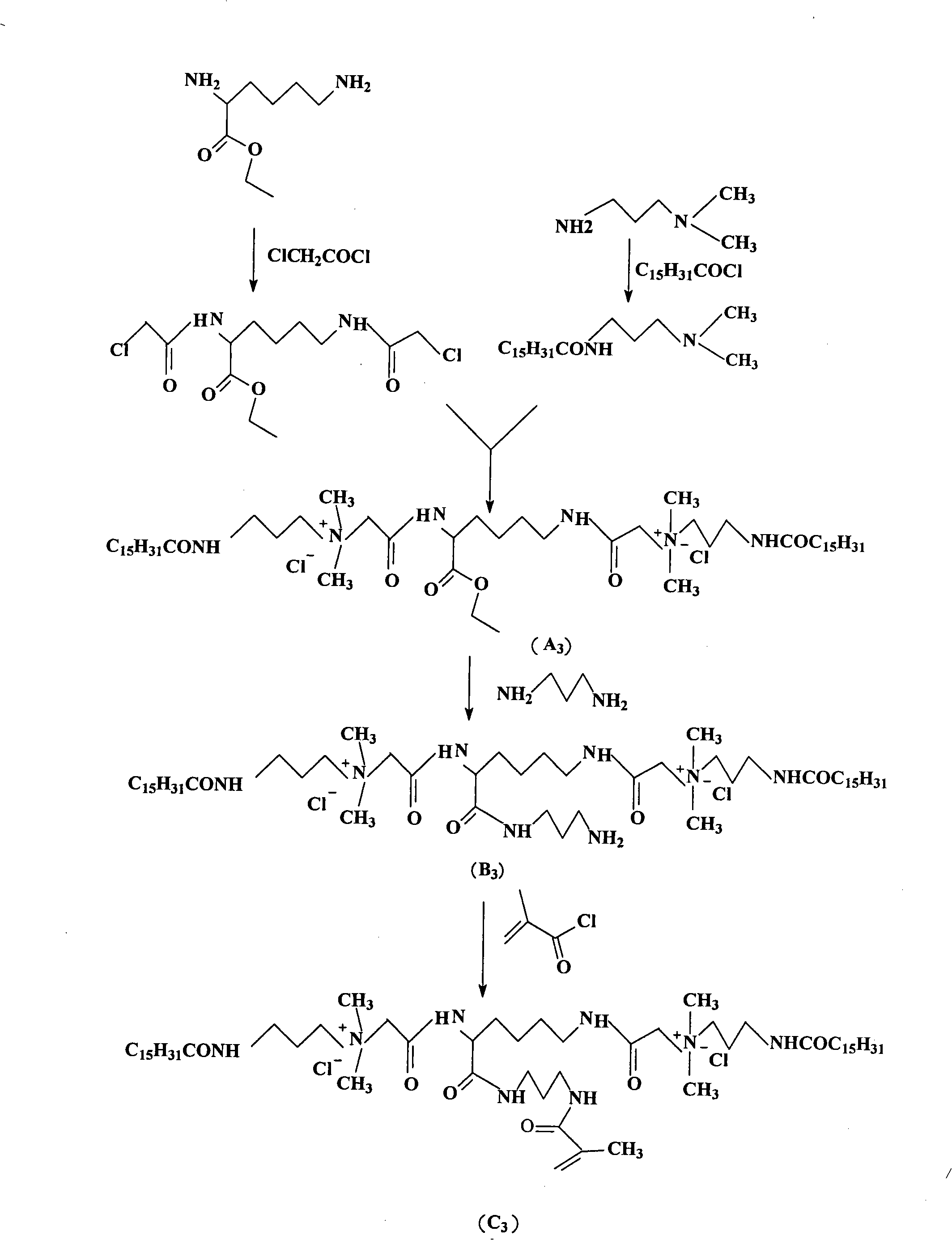
[0088] In this example, acryloyl bis-quaternary ammonium salt (C3) was prepared.
[0089] (1) 2, the preparation of 6-two (2-chloroacetamide) ethyl hexanoate
[0090] Dissolve 17.4 grams (0.1mol) of ethyl 2,6-diaminocaproate in 100ml of anhydrous dichloromethane, then add 22.2 grams (0.22mol) of triethylamine, lower the temperature to 0°C, and dissolve in 150ml Add 24.9 grams (0.22mol) of anhydrous dichloromethane (0.22mol) chloroacetyl chloride dropwise into the ethyl 2,6-diaminocaproate solution, and react for 2 hours after the dropwise addition; filter off the triethylamine salt, collect the dichloromethane layer, and Wash with dilute hydrochloric acid, saturated sodium bicarbonate solution, saturated sodium chloride, and deionized water successively, dry over anhydrous sodium sulfate, filter, and distill off dichloromethane under reduced pressure, and crystallize to obtain 2,6-bis(2-chloroacetamide ) ethyl hexanoate 31.1g, yield 95.0%.
[0091] Ms(+) theoretical: 326, 32...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com