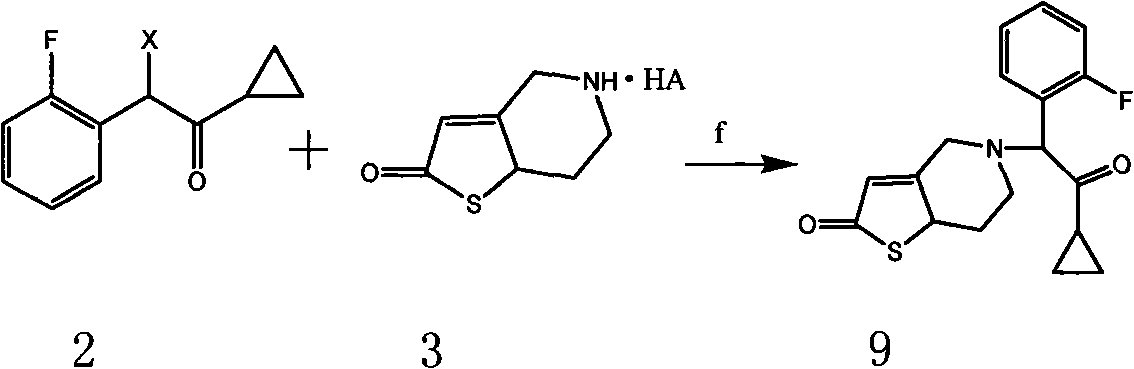
Preparation method for hydrogenated pyridine derivant and its salt
A technology for hydrogenating pyridine and derivatives, which is applied in the field of medicine and chemical industry, and can solve the problems of many steps, unfavorable industrialized large-scale production, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
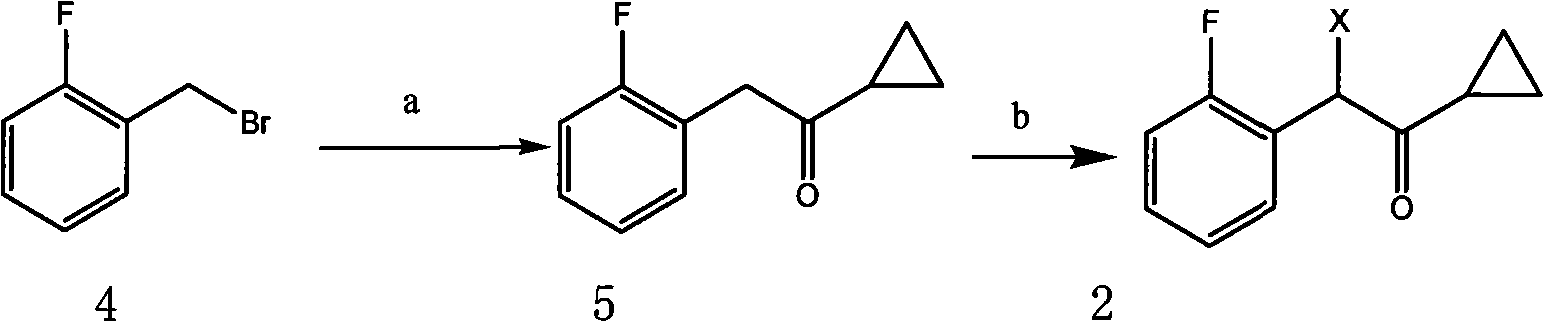
[0037] The preparation of embodiment 1 cyclopropyl-2-fluorobenzyl ketone
[0038]In 100ml of anhydrous tetrahydrofuran solution containing 14.4g (0.6mol) of magnesium strips, add dropwise a mixed solution of 104g (0.55mol) of 2-fluorobenzyl bromide and 400ml of anhydrous tetrahydrofuran while stirring, after the dropwise addition, stir at room temperature 1 hour, then stirred and heated to reflux for 2 hours, the reaction was completed, and after being placed at room temperature, a mixed solution of 37g (0.55mol) cyclopropyl cyanide and 250ml anhydrous tetrahydrofuran was added dropwise with stirring in the reaction system. After 1 hour, heat and reflux and stir the reaction for 2 hours. After the reaction is over, slowly add 300 ml of saturated ammonium chloride aqueous solution dropwise to the reaction system, and then use CH 2 Cl 2 Extraction, the extraction solution was sequentially washed with saturated NaHCO 3 After washing with aqueous solution and saturated brine, dr...
Embodiment 2
[0041] The preparation of embodiment 2α-cyclopropylcarbonyl-2-fluorobenzyl bromide
[0042] Dissolve 68 g (0.38 mol) of cyclopropyl-2-fluorobenzyl ketone obtained in Example 1 in 800 ml of dichloromethane, and add 64 g (0.4 mol) of bromine dropwise under stirring. After the dropwise addition, reflux and stir to react 8 hour, after the reaction was completed, the reaction solution was slowly poured into an ice-water mixture for liquid separation, and the water layer was washed with CH 2 Cl 2 Extraction, combined organic layer, successively with saturated NaHCO 3 aqueous solution and saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure, and the obtained oil was purified by silica gel column chromatography (eluent: petroleum ether: ether = 3:1) to obtain α-cyclopropane 76 g (0.29 mol) of carbonyl-2-fluorobenzyl bromide, yield 77.5%.
[0043] 1 H NMR (CDCI 3 )δppm: 0.91-0.99 (2H, m), 1.00-1.15 (2H, m), 2.11-2.13 (1H, m), ...
Embodiment 3
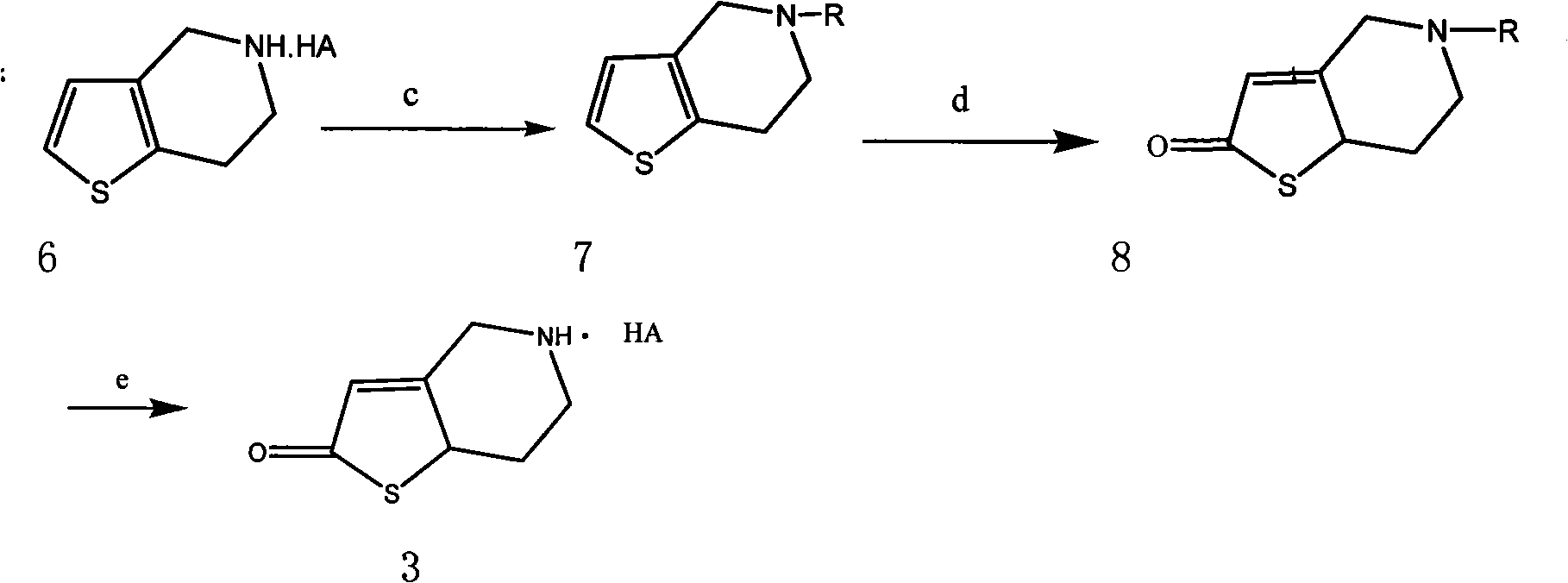
[0045] The preparation of embodiment 3N-triphenyl-4,5,6,7-tetrahydrothieno[3,2-C]pyridine
[0046] 17.5g (0.1mol) of 4,5,6,7-tetrahydrothieno[3,2-C]pyridine hydrochloride was dissolved in 100ml of dichloromethane, and 4g (0.1mol) of NaOH was added dropwise at room temperature to dissolve in The solution in 50ml of water was added dropwise, then stirred and reacted at room temperature for 2 hours, separated, the organic layer was washed with 1mol / ml dilute hydrochloric acid aqueous solution, and then washed with saturated NaHCO 3 After washing with aqueous solution and saturated brine, dry with anhydrous sodium sulfate for 4 hours, filter, add 14ml of triethylamine, add 27.9g of triphenylchloromethane and 50ml of CH 2 Cl 2 The solution was added dropwise, reacted at room temperature for 6 hours, separated, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. A viscous liquid was obtained, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com