Synthesis of novel organic luminescent material containing triphenylethylene carbazole derivant structure and application thereof
A technology of triphenylene carbazole and luminescent materials, applied in luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of short life, low glass transition temperature, and low luminous efficiency, and achieve high glass transition temperature , simple synthesis process, high luminous intensity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The synthesis method of above-mentioned luminescent material, comprises the following steps:
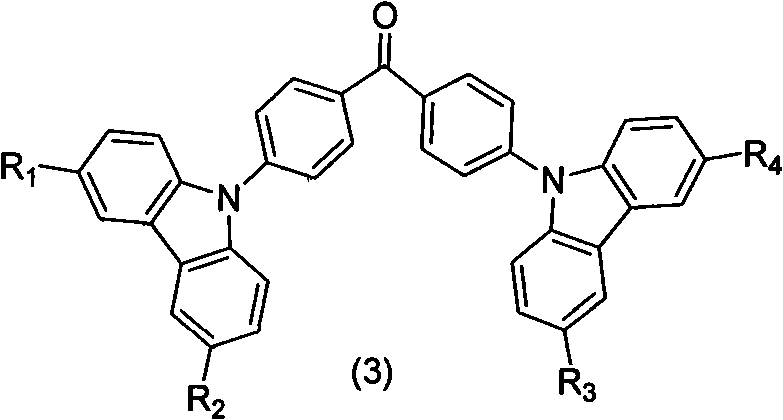
[0021] The first step: the synthesis of carbazolyl benzophenone
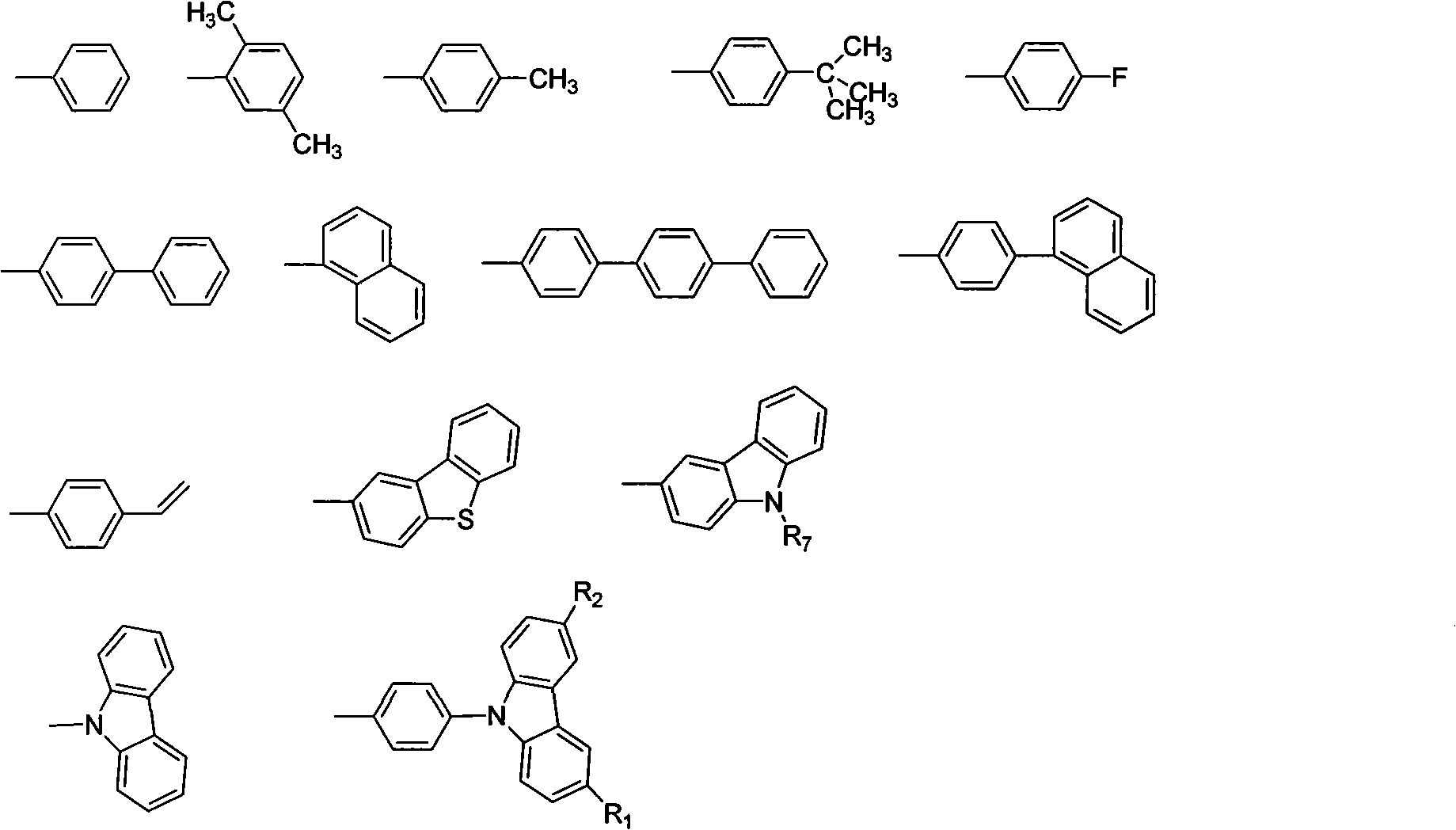
[0022] The synthesis of carbazolyl benzophenone is obtained by reacting 4-halogenated benzophenone with carbazole and substituent carbazole. In the present invention, 4-fluorobenzophenone is preferably used as the raw material for preparing carbazolyl-containing benzophenone. The preparation process is simple and the yield is high, which is one of the main features of the present invention. For the synthesis of the substituent carbazole starting material, according to the mentioned R 1 , R 2 , R 3 , R 4 The structure of the substituent is synthesized by conventional organic synthesis methods, including Friedel-Crafts alkylation, halogenation, Suzuki reaction, Heck reaction, Wittig reaction, etc.
[0023] Step 2: Conversion of ketone carbonyl to double bond
[0024] Both Wittig and Wittig-Horner methods can...
Embodiment 1
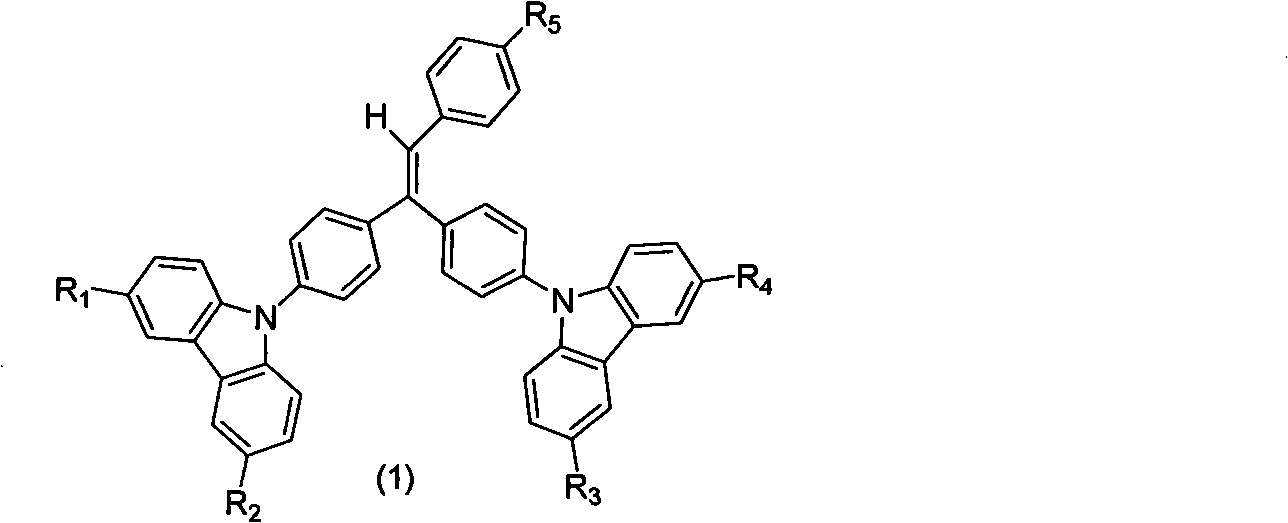
[0030] 9,9'-(4,4'-(2-(4-(1-naphthyl)phenyl)-1,1-vinyl)bis(4,1-phenylene))bis(9H-carba azole) synthesis:
[0031] (1) Synthetic intermediate bis(4-(9H-carbazolyl)phenyl)methanone
[0032] 25g (0.15mol) of carbazole was dissolved in 200mL of DMF, 20g (0.19mol) of potassium tert-butoxide was added under stirring and the temperature was raised to 60°C for 30min, then 14.8g (0.068mol) of 4,4'-difluorobenzophenone was added ), and then heated to 110 ° C for 12 h. Stop the reaction, pour it into 500mL water after cooling to produce a precipitate, filter it with suction, and wash it with water several times. Dissolve the solid in 150mL of dichloromethane to form a solution, add an appropriate amount of anhydrous sodium sulfate to dry, and filter. 150 mL of acetone was added to the filtrate, dichloromethane and most of the acetone were evaporated by a rotary evaporator to obtain a precipitate, filtered by suction, washed 3 times with a small amount of acetone, and vacuum-dried to ob...
Embodiment 2
[0041] Synthesis of 9,9'-(4,4'-(2-(4-terphenyl)-1,1-vinyl)bis(4,1-phenylene))bis(9H-carbazole) Method Referring to Example 1, the boric acid used is 4-biphenylboronic acid. The pure product is an off-white powder with a yield of 62%, λ max em It is 457nm, and Tg is 135°C.
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com