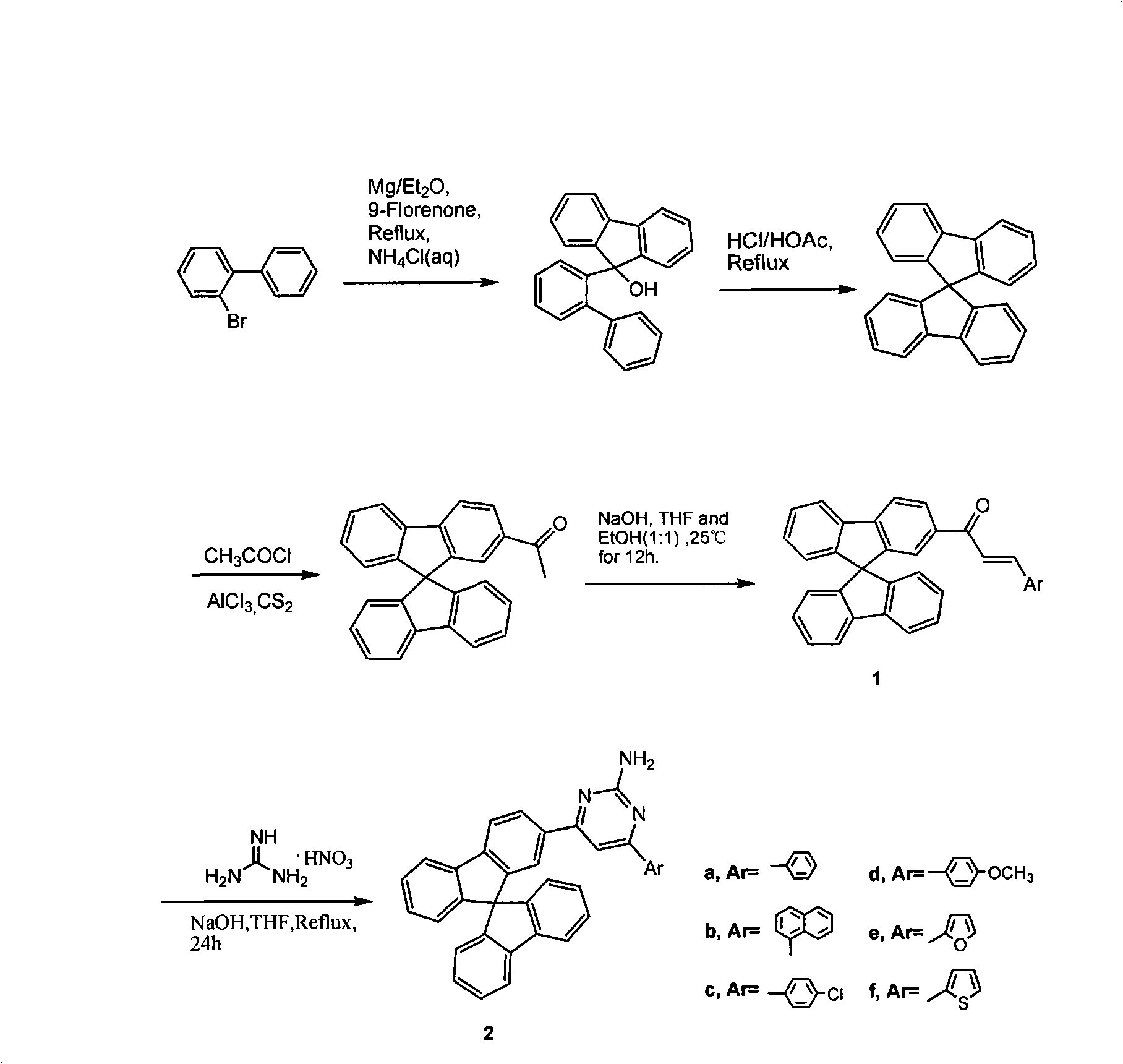
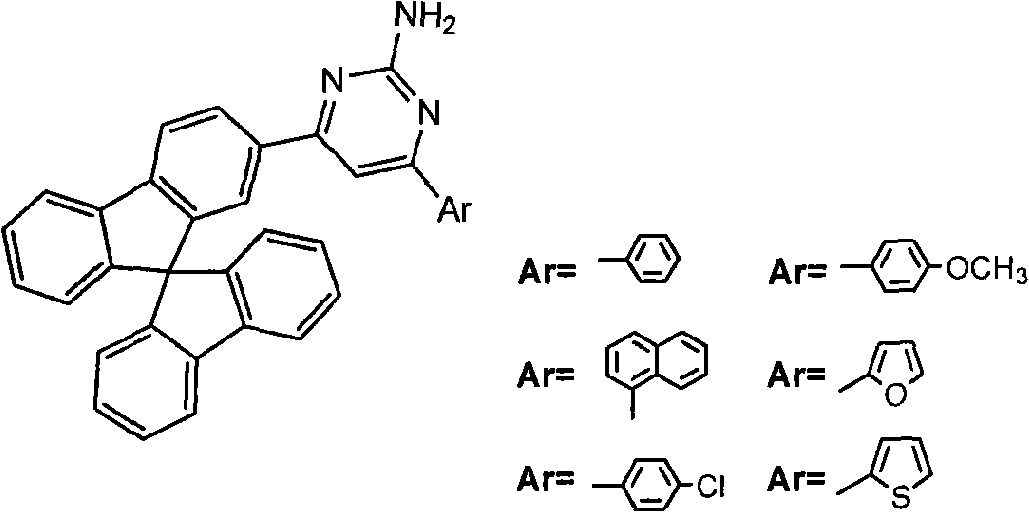
Aryl substituted pyrimidine spirobifluorene derivative and preparation thereof
A technology of pyrimidine spirobifluorene and aryl pyrimidine, which is applied in the field of aryl-substituted pyrimidine spirobifluorene derivatives and their preparation, can solve the problems of not being developed, achieve novel molecular structure, good thermal stability, and improve optical purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Preparation of spirobifluorene
[0022] Add 1.26g of magnesium to a flask containing 10ml of ether, and slowly add a solution of 2-bromobiphenyl (11.65g, 50.0mmol) in 20ml of ether under the trigger of 1,2-dibromoethane, and reflux for 3 hours , to make the Grignard reagent. The prepared Grignard reagent was slowly added to a 40ml solution of 9-fluorenone (9.9g, 55mmol), and refluxed for 3 hours. The resulting yellow magnesium complex was collected by suction filtration, washed with dry ether, and the solid was stirred in ice-cold saturated ammonium chloride solution. After 2 hours, the solid was collected, filtered and dried, dissolved in hot acetic acid, and added dropwise Concentrate hydrochloric acid until the generated solids no longer increase, filter, and recrystallize from ethanol to obtain 14.0 g of spirobifluorene in colorless flaky crystals, with a yield of about 88.7%. m.p.207~208℃; 1 HNMR (CDCl 3 , 500MHz) δ: 6.75(d, J=7Hz, 1H, Ar-H), 7.13(t, J=8Hz,...
Embodiment 2
[0040] (1) Preparation of spirobifluorene
[0041] Same as Example 1
[0042] (2) Preparation of 2-acetylspirobifluorene
[0043] Same as Example 1
[0044] (3) preparation of chalcone
[0045] Add 2-acetyl spirobifluorene (1.58g, 5mmol) and 1-naphthaldehyde (0.80g, 5.1mmol) and tetrahydrofuran and ethanol in the volume ratio of 4: 1 mixed solvent 100ml, and then add 10ml of 30% sodium hydroxide solution was stirred at room temperature for 12 hours, acidified to neutrality with 2N hydrochloric acid, extracted with dichloromethane (3×20ml), then dried with anhydrous magnesium sulfate, spin-dried and washed with ethanol After recrystallization, 1.99 g of chalcone as a pale yellow solid was obtained with a yield of about 80%.
[0046] Physical constants and spectral data of the product:
[0047] Product appearance: pale yellow solid, melting point: 237-239°C, 1 HNMR (CDCl 3 , 500MHz) δ: 6.77(tJ=8Hz3H), 7.14(t, J=8Hz, 2H), 7.20(t, J=8Hz, 2H), 7.50(m, 6H), 7.56(q, 2H), 7.81(...
Embodiment 3
[0055] (1) Preparation of spirobifluorene
[0056] Same as Example 1
[0057] (2) Preparation of 2-acetylspirobifluorene
[0058] Same as Example 1
[0059] (3) preparation of chalcone
[0060] Add 2-acetylspirobifluorene (1.58g, 5mmol) and p-chlorobenzaldehyde (0.72g, 5.1mmol) and tetrahydrofuran and ethanol into the flask in a volume ratio of 4: 1 mixed solvent 100ml, and then add 10ml of 30% sodium hydroxide solution was stirred at room temperature for 12 hours, acidified to neutrality with 2N hydrochloric acid, extracted with dichloromethane (3×20ml), then dried with anhydrous magnesium sulfate, spin-dried and washed with ethanol After recrystallization, 2.07 g of chalcone in colorless flaky crystals was obtained with a yield of about 86%.
[0061] Physical constants and spectral data of the product:
[0062]Product appearance: colorless flaky crystal, melting point: 190~192℃; 1 HNMR (CDCl 3 , 500MHz) δ: 6.76(m, 3H), 7.13(t, J=8Hz, 2H), 7.19(t, J=8Hz, 1H), 7.34(q, 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com