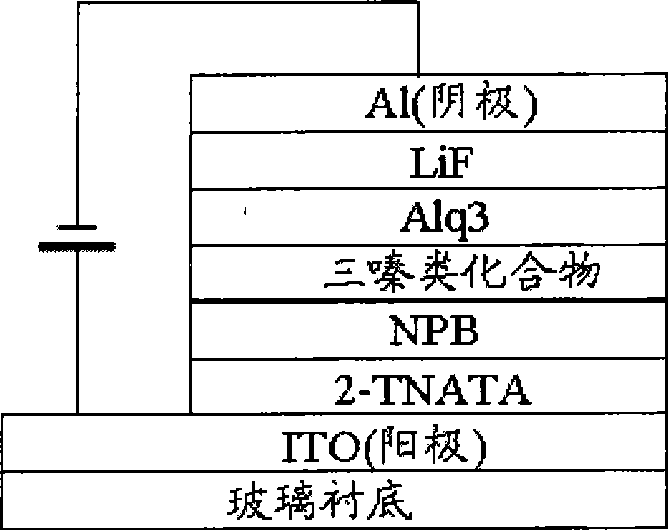
S-triazine derivates with white light and preparation method and application thereof
A technology of s-triazine and derivatives, which is applied in the fields of white light-emitting s-triazine derivatives and their preparation and application, can solve the problems of high driving voltage, low device energy efficiency, poor stability and the like, and achieves low steric hindrance, Conjugated, low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
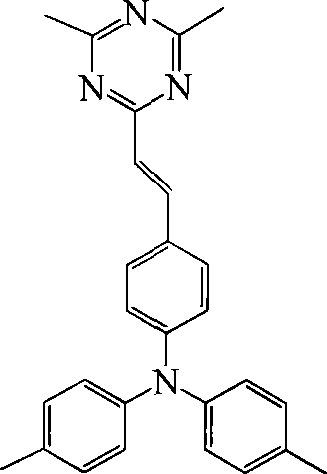
[0040] Example 1: Synthesis of 2,4-dimethyl-6-[4-(N,N-di-p-tolyl)-anilinoethyl]-s-triazine (1)
[0041] Weigh 1 molar amount of 1,3,5-trimethyl-s-triazine and 0.6 molar amount of potassium hydroxide, then add 6 L of methanol to dissolve. At the reflux temperature, 3 L of methanol solution of 1 molar amount of 4-(N,N-xylanilino)benzaldehyde was dripped dropwise into the flask. Solvent, the residue was separated by silica gel column chromatography with V(toluene):V(ethanol)=10:1 toluene-ethanol mixture as eluent to obtain a pale yellow product. Yield 41.3%, mass spectrum m / z: 406.1 (M + ). 2,4-Dimethyl-6-[4-(N,N-di-p-tolyl)-anilinoethyl]-s-triazine (1) has an exact molecular mass of 406.22, which was measured by a mass spectrometer in this embodiment The molecular mass is 406.1, indicating that the compound (1) obtained in Example 1 is correct.
Embodiment 2
[0042] Example 2: Synthesis of 2-methyl-4,6-bis[4-(N,N-di-p-tolyl)-anilinoethyl]-s-triazine (2)
[0043] Weigh 1,3,5-trimethyl-s-triazine, 1.2 moles of potassium hydroxide, and 2 moles of 4-(N,N-dimethylanilino)benzaldehyde, then add methanol 20L dissolve. Reflux for 12 hours, remove the solvent with a rotary evaporator, and use a V(toluene):V(ethanol)=10:1 toluene-ethanol mixture as the eluent to separate the residue through silica gel column chromatography to obtain a pale yellow product. Yield 38.6%, m / z: 690.3 (M+H) + ; 2-methyl-4,6-bis[4-(N,N-di-p-tolyl)-anilinoethyl]-s-triazine (2) exact molecular mass is 689.35), measured m / z: 690.3( M+H) + , indicating that the obtained compound (2) is correct.
Embodiment 3
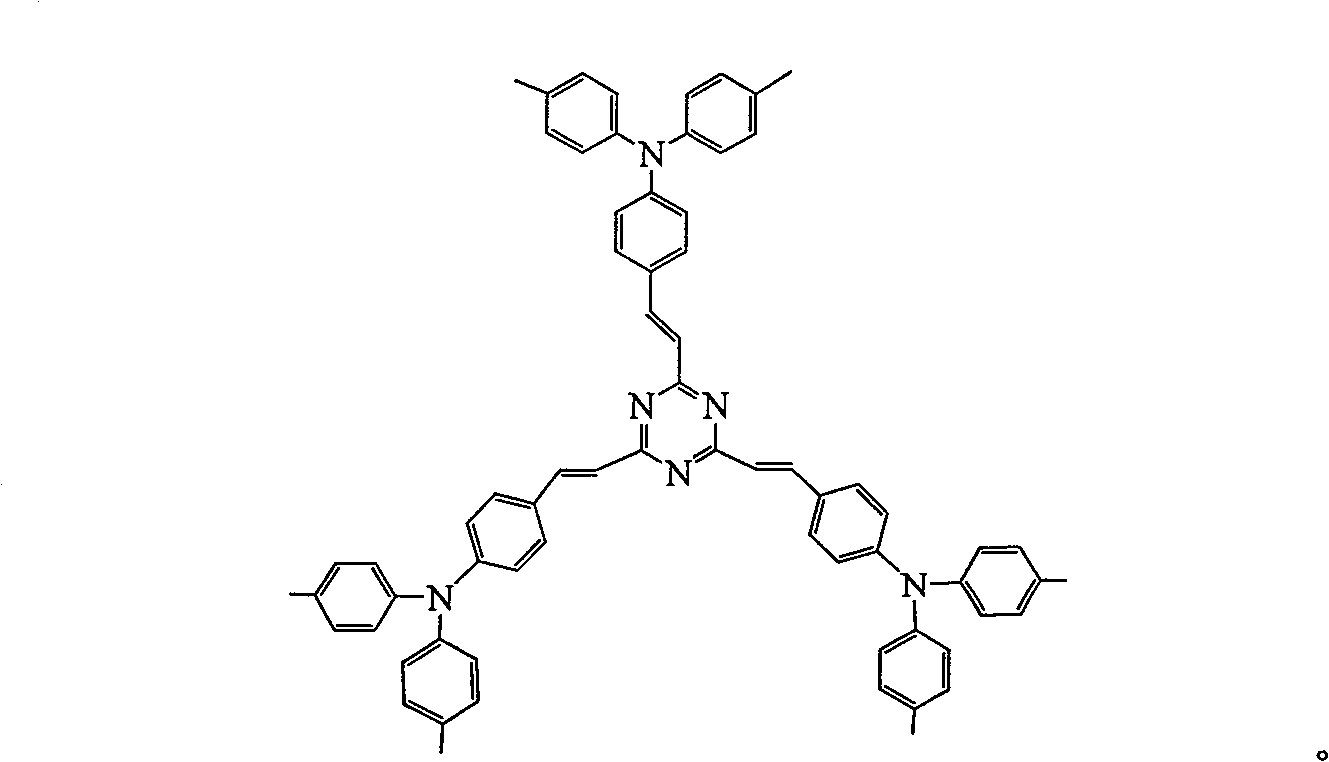
[0044] Example 3: Synthesis of 2,4,6-three [4-(N,N-di-p-tolyl)-anilinoethyl]-s-triazine (3)
[0045] Weigh 6 moles of 4-(N,N-xylanilino)benzaldehyde and 1.8 moles of potassium hydroxide, then add 20 L of methanol to dissolve. 5 L of 1,3,5-trimethyl-s-triazine solution in which 1 mole was dissolved was dropped dropwise into the flask. Toluene): V (ethanol)=10:1 toluene-ethanol mixture as eluent, separated by silica gel column chromatography to obtain light yellow product. Yield 43.7%, m / z: 973.5 (M+H) + . The exact molecular mass of 2,4,6-tris[4-(N,N-xylyl)-anilinoethyl]-s-triazine (3) is 972.49, measured m / z: 973.5 (M+H) + , illustrates that the compound (3) obtained in Example 3 is correct.
PUM
| Property | Measurement | Unit |
|---|---|---|
| luminous efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com