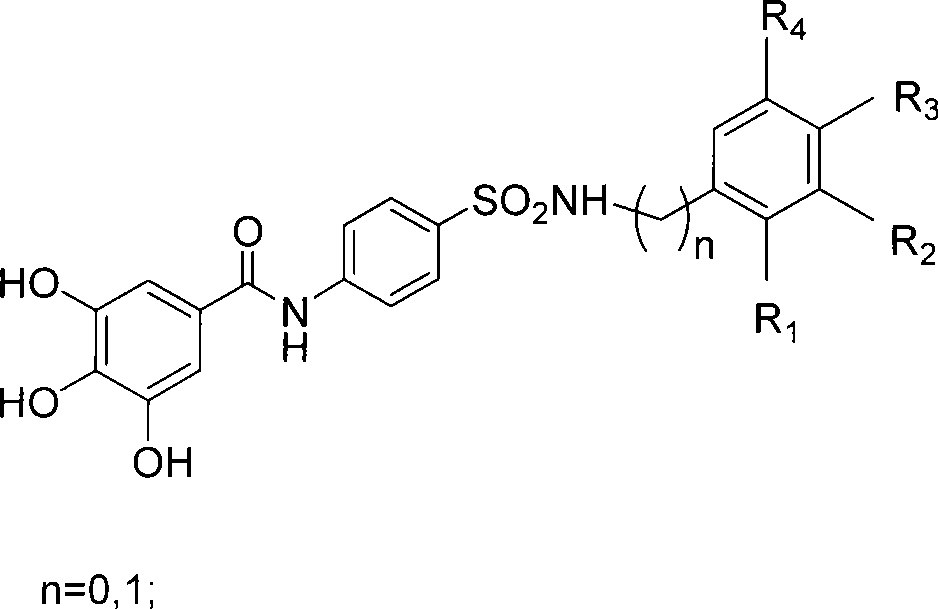
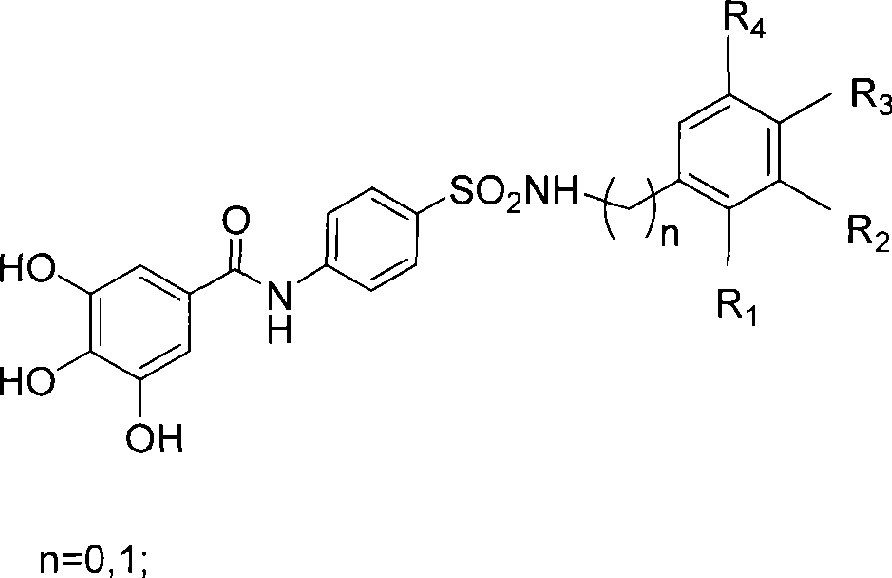
Nitrogen-containing polyhydroxy fragrant compounds, preparation and uses thereof
A compound and polyhydroxyl technology, which is applied in the field of nitrogen-containing polyhydroxyaromatic compounds and their preparation, can solve the problems of incomplete eradication of viruses in the body, high price, and limited efficacy, and achieve the effect of improving integrase inhibitory activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
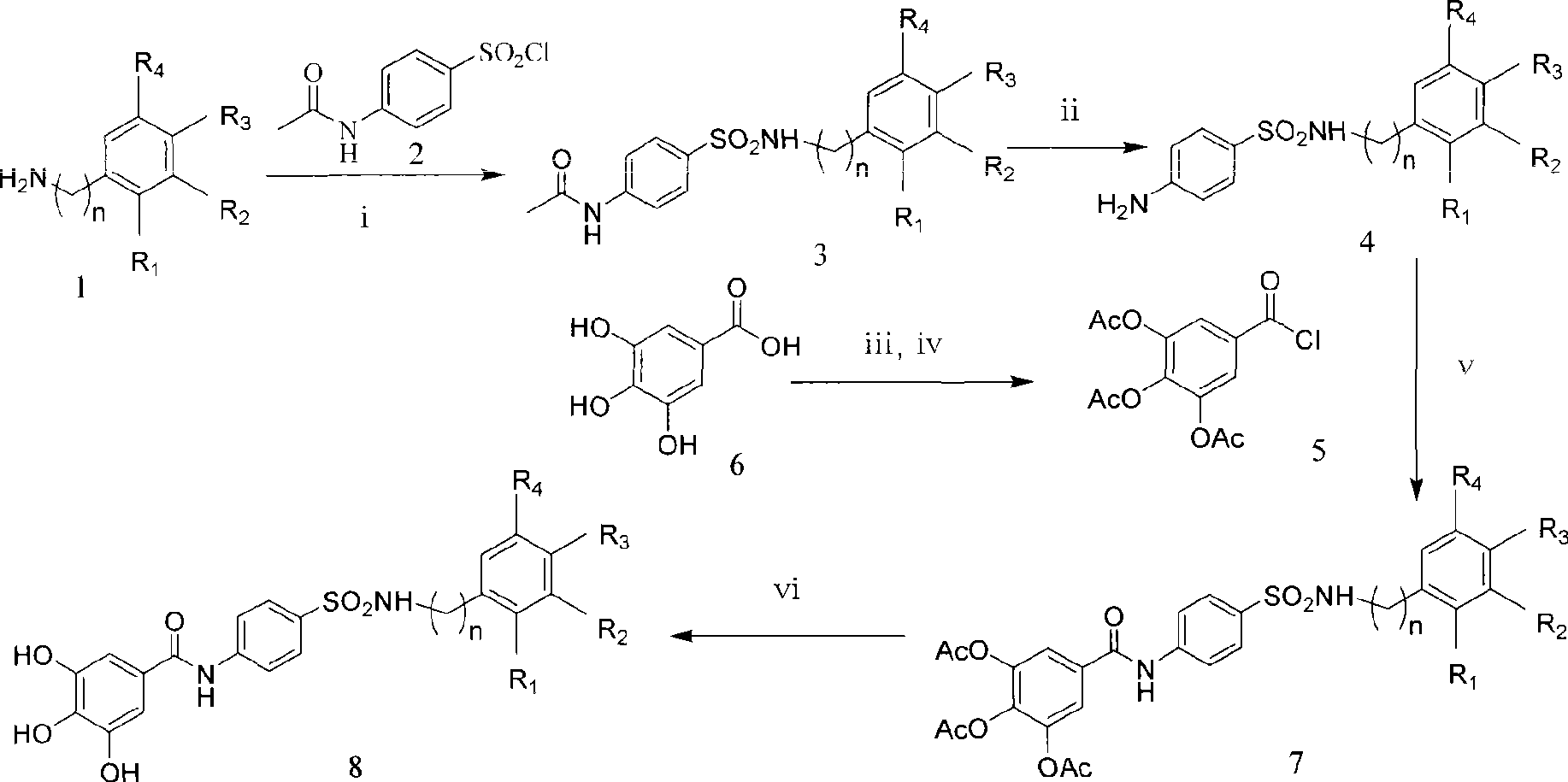
[0035] Example 1. Preparation of 4-(N-substituted phenyl or benzyl-amino)-sulfa-1-aniline 4
[0036]Add 120.0mmol of substituted aniline or benzylamine and dry pyridine (10mL, 123.9mmol) into a 100mL three-necked flask equipped with a drying tube, add 216.0mmol of p-acetamidobenzenesulfonyl chloride in batches under ice bath conditions, and stir after addition. React for 10min, remove the ice bath, stir at room temperature for 4h, add distilled water (100mL), stir for 20min, precipitate a solid, filter, wash the filter cake three times, dissolve the solid with 10% sodium hydroxide solution, filter, and use 18% hydrochloric acid solution for the filtrate Adjust pH 3~4, precipitate solid, filter, wash filter cake three times, and dry to obtain compound 3. Add compound 3 into a 50mL round bottom flask, add sodium hydroxide solution (20mL) and methanol (12mL) at a concentration of 5mol / L , The reaction was stirred at 70°C for 3 hours, the pH was adjusted to 6 with a hydrochloric acid ...
Embodiment 23
[0040] Example 2.3 Preparation of 4,5-Triacetylgalloyl Chloride 5
[0041] Add gallic acid 6 and ethyl acetic anhydride with a molar ratio of 1:3 to 10 and 5.0 mL of pyridine into a 50 mL round bottom flask. Stir and react for 20 hours at 25°C. Add 200 mL of ether or water to the reaction solution and let it stand. The white precipitate is separated out, filtered, recrystallized with acetone / cyclohexane, filtered and dried to obtain the intermediate; add the intermediate and thionyl chloride with a molar ratio of 1:2~10 to 50mL equipped with a drying tube and a condenser tube In a round bottom flask, heated in an oil bath, stirred at 70-85°C for 3-10 hours, and evaporated under reduced pressure at 40°C to remove the solvent to obtain compound 5, which was diluted by adding 10 mL of acetone for later use.
Embodiment 33
[0042] Example 3.3 Preparation of 4,5-triacetoxy-N-(4-(N-substituted aniline or benzylaminosulfonyl)phenyl)benzamide 7
[0043] Add 4-(N-substituted aryl-amino)-sulfa-1-aniline 4, 10 mL of dry acetone and pyridine to a 100 mL three-necked flask equipped with a drying tube and a constant pressure dropping funnel, and slowly under ice Add acid chloride 5 prepared in Example 2 above, the molar ratio of acid chloride 5 to compound 4 is 1:1 to 2; the molar ratio of acid chloride 5 to pyridine is 1:1 to 2 in acetone, and the reaction is completed in about 1 hour. 10min, remove the ice bath, stir at room temperature for 15-30h, filter, evaporate the solvent under reduced pressure to obtain an oily substance, add 80mL of distilled water and stir to precipitate a solid, filter and dry; the crude product is purified by silica gel column chromatography, petroleum ether / ethyl acetate gradient For elution, collect the combined product eluent, evaporate the solvent under reduced pressure, add a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com