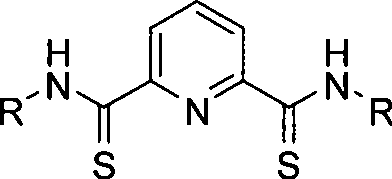
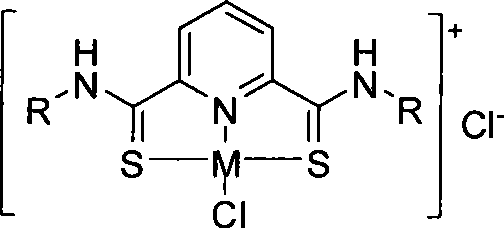
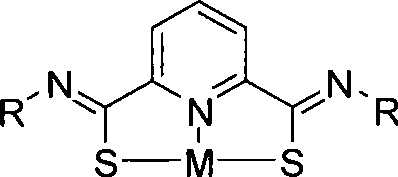
Forcipate thioacid amide ligand, complex compound and application of sulfo-2, 6-pyridine diformamide framework
A technology of pyridinedicarboxamide and pincer thioamide, which is applied in the field of pincer thioamide ligands and their complexes, to achieve the effects of amplification reaction, good structural stability, and easy adjustment and modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of thio-2,6-pyridinediformamide
[0040] Add 120mL of thionyl chloride into the drained 250mL three-necked flask, add 2,6-pyridinedicarboxylic acid powder (0.4mol, 66.81g) under nitrogen flow, stir evenly while adding, add 1ml N, N -Dimethylformamide catalyzed the reaction and heated to reflux; at the beginning of reflux, 2,6-pyridinedicarboxylic acid was not completely dissolved in thionyl chloride to form a white suspension. As the reaction progressed, a large amount of gas was generated and entered Exhaust gas treatment device, the product 2,6-pyridine diacid chloride can be completely dissolved, so when the solution becomes clear and no gas is generated, it indicates that the reaction is basically complete; vacuum distillation removes excess thionyl chloride to obtain a crude product that can be directly Put into the next step reaction.
[0041] In a 250mL round bottom flask, weigh 20.4g (0.10mol) of 2,6-pyridinedicarboxylic acid chloride, add 50mL of dichloro...
Embodiment 2
[0044] Synthesis of thio-2,6-pyridinedicarboxylic acid butylamide
[0045] In a 500mL round bottom flask, weigh 2,6-pyridinedicarbonyl chloride (0.15mol, 30.6g), add 100mL of acetonitrile, stir well, add 62.5mL of triethylamine (0.45mol, 45.5g), and then add n-butyl Amine 37mL (0.375mol, 27.4g), the reaction exotherms, and ice water is used for cooling. After the reaction is not exothermic, heat to make the reaction react at 50-60°C; point plate monitoring, when the reaction is complete, the system is spin-dried first, the obtained solid is ground, and then the volume ratio of ethanol and water in 100mL (3: Suspend in the mixed solution of 1), stir in a water bath at 50-60°C for 10-30 minutes, filter with suction, and wash once with cold ethanol to obtain 17.9 g of pure product 2,6-pyridinedicarboxylic acid butylamine, yield: 73 %.
[0046] Add 10.0g (0.036mol, 1eq) of 2,6-pyridinedicarboxylic acid butylamine weighed and 17.25g (0.0425mol,) of Lawesson's reagent into a 100mL Schle...
Embodiment 3
[0049] Synthesis of thio-2,6-pyridinedicarbonyl(2,4,6-trimethylaniline)amine
[0050] In a 250mL round-bottom flask, weigh 20.4g (0.1mol) of 2,6-pyridinedicarbonyl chloride, add 50mL of dichloromethane, stir well, add 30.3g (0.3mol) of triethylamine, and then add 2,4, 29.7 g (0.22 mol) of 6-trimethylaniline, the reaction exotherms, and ice water is used for cooling. When the reaction does not exotherm, the reaction is refluxed; monitoring by the dot plate, when the reaction is complete, the system is spin-dried first, the obtained solid is ground, and then suspended in a mixed solution of 70 mL (ethanol and water volume ratio 3: 1), Stir in a water bath at 50-60°C for 10-30 minutes, filter with suction, and wash once with cold ethanol to obtain 30.60g of pure 2,6-pyridinedicarbonyl (2,4,6-trimethylphenyl)amine, yield 76.2%.
[0051] Add 4.015g (0.01mol) of the weighed 2,6-pyridinedicarbonyl (2,4,6-trimethylphenyl)amine and 4.449g (0.011mol) of Lawesson's reagent to 50mL Schlenk un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com