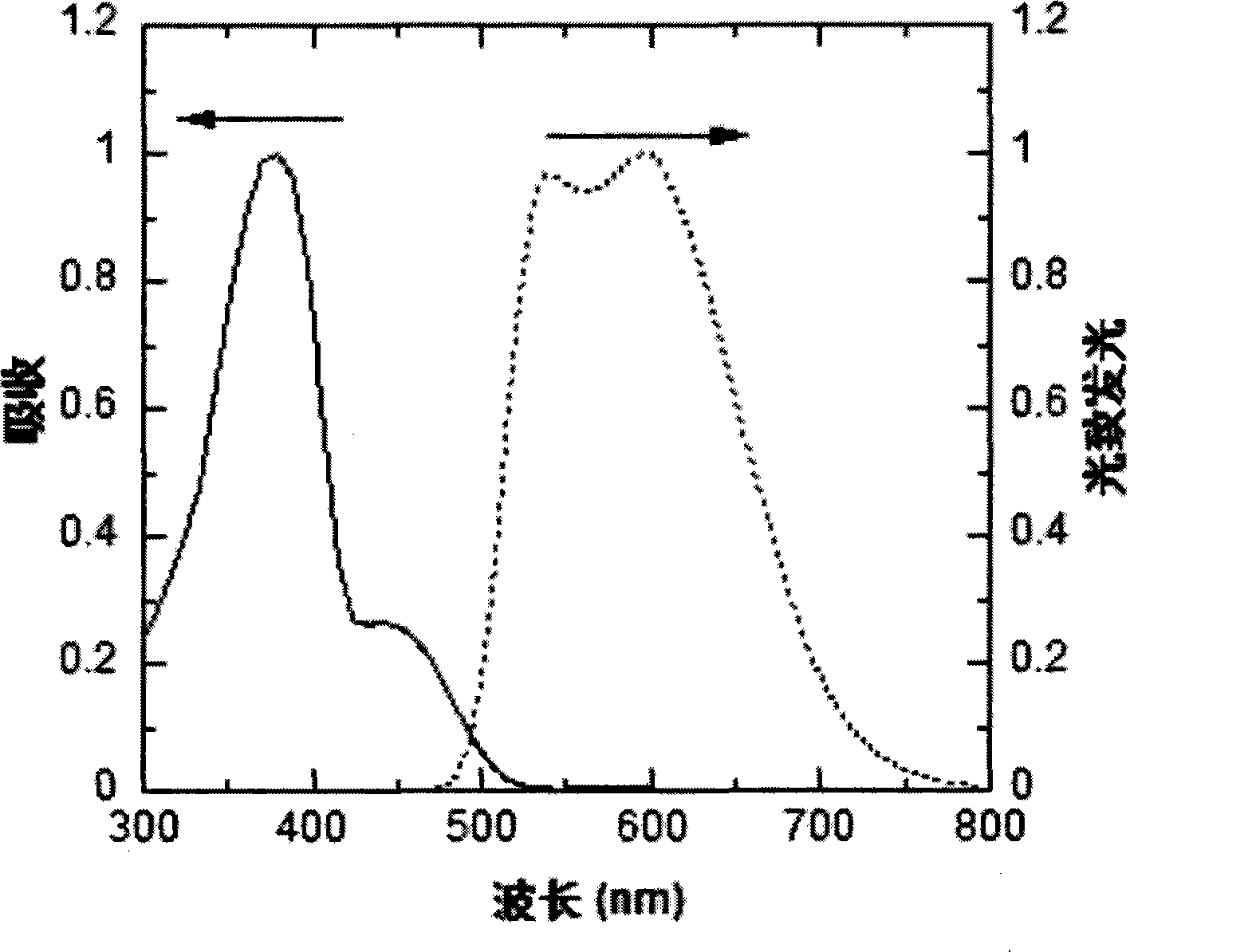
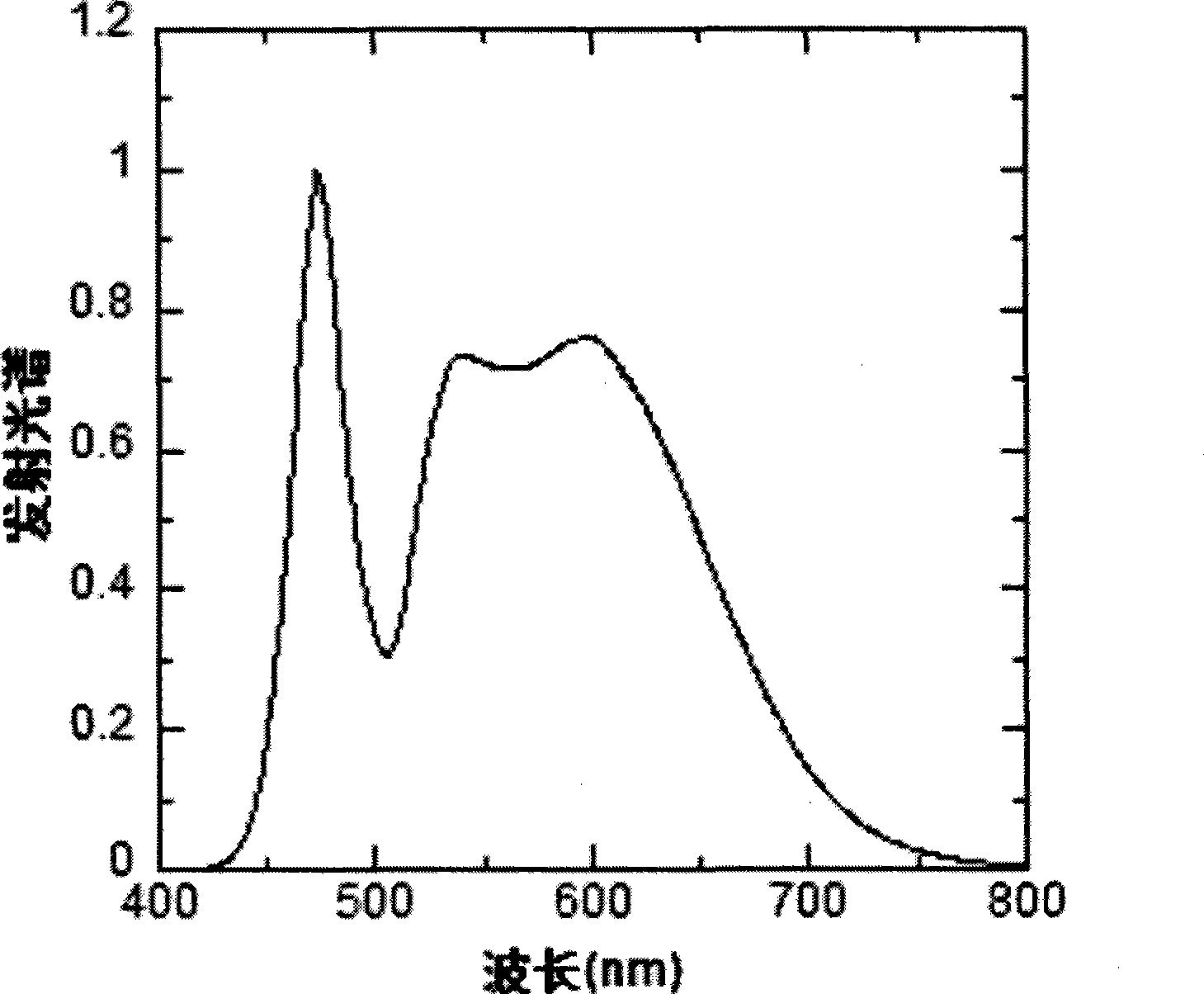
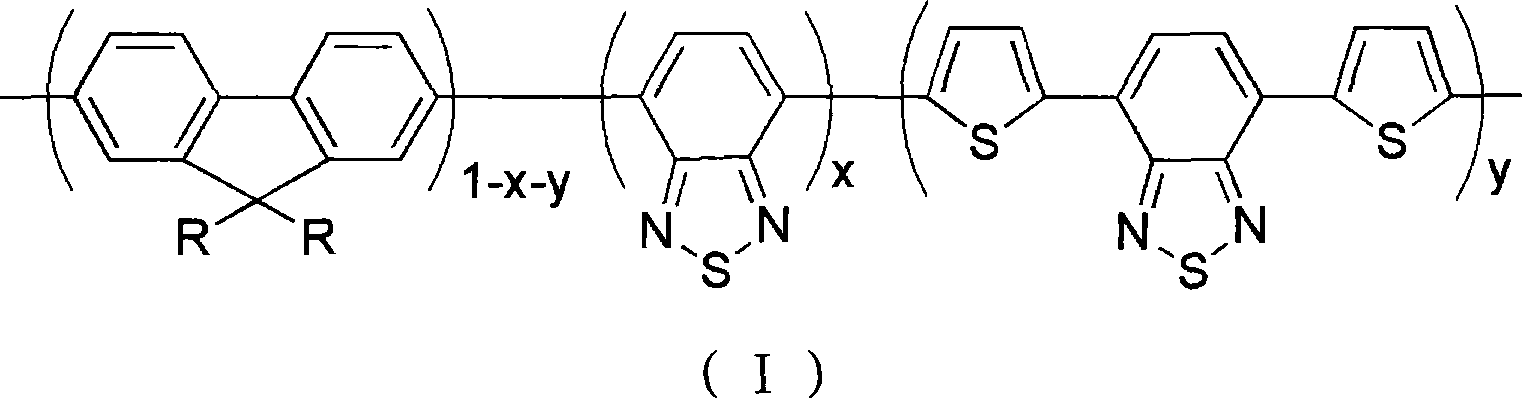
Red and green light emitting conjugated polymer and preparation method as well as application thereof
A conjugated polymer, red and green light technology, applied in chemical instruments and methods, luminescent materials, gas discharge lamps, etc., can solve the problems of polymer molecular chain aggregation, unstable emission spectrum, etc., and achieve stable film morphology , The effect of simple material synthesis and preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of 2,7-dibromofluorene.
[0023] Prepare 2,7-dibromofluorene according to the method disclosed in World Patent WO 99 05184 in 1997 and the method disclosed in "Chem.Mater" (Chem. Powder (80 mg, 1.43 mmol) was poured into a three-necked flask, 100 ml of chloroform was added, cooled in an ice-water bath, a mixed solution of bromine (31.4 g, 0.196 mol) and 35 ml of chloroform was added dropwise from a constant pressure funnel, The temperature in the bottle should not exceed 5°C during the dropwise addition. After the reaction is completed, filter and recrystallize with chloroform to obtain white crystals. 13 C NMR and GC-MASS tests showed that it was the target product 2,7-dibromofluorene.
Embodiment 2
[0025] Preparation of 2,7-dibromo-9,9-dialkylfluorene
[0026] Take the preparation of 2,7-dibromo-9,9-di-n-octylfluorene as an example to illustrate. 2,7-dibromo-9,9-di-n-octylfluorene was prepared according to the method disclosed in World Patent WO9905184 in 1997 and the method disclosed in "Chem. Mater" (Chem. Mater) 11 (1997) 11083. Get 2,7-dibromofluorene (15.0 g, 0.046 mol) obtained in Example 1, and pour benzyltriethylammonium chloride (0.09 g, 0.39 mmol) into a three-necked flask, and add 150 ml of dimethyl sulfoxide , 45 milliliters of NaOH aqueous solution with a weight ratio of 50%, was stirred vigorously at room temperature to form a suspension, 1-bromo-n-octyl alkane (18.6 g, 0.097 mmol) was added dropwise, stirred continuously for 3 hours, then extracted with diethyl ether, and diethyl ether was combined phase, washed with saturated aqueous NaCl, anhydrous MgSO 4 dry. Recrystallization from acetone gave white crystals after removal of the solvent. 13 C NMR a...
Embodiment 3
[0029] Preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-9,9-dialkylfluorene
[0030] The preparation of 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl)-9,9-di-n-octylfluorene is taken as an example for illustration. 2,7-bis(4,4,5,5-tetramethyl-1,3,2-dioxaborolane-diyl) was prepared according to the method disclosed in "Macromolecules" 30 (1997) 7686 -9,9-Di-n-octylfluorene. The dried 2,7-dibromo-9,9-dioctylfluorene (10.96 g) obtained in Example 2 was dissolved in 180 ml of tetrahydrofuran (THF), and 1.6 mol / liter of n-butyl was added dropwise at -78°C Lithium 28 ml, reacted under nitrogen atmosphere for 2 hours, then quickly added 2-isopropyl-4,4,5,5-tetramethyl-1,3,2-dioxaborane 25 ml, reacted at low temperature After 2 hours, slowly rise to room temperature and react for 24 hours. The reaction mixture was poured into water and extracted with ether. The organic layer was washed with brine and washed with anhydrous MgSO 4 dry. After removing the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com