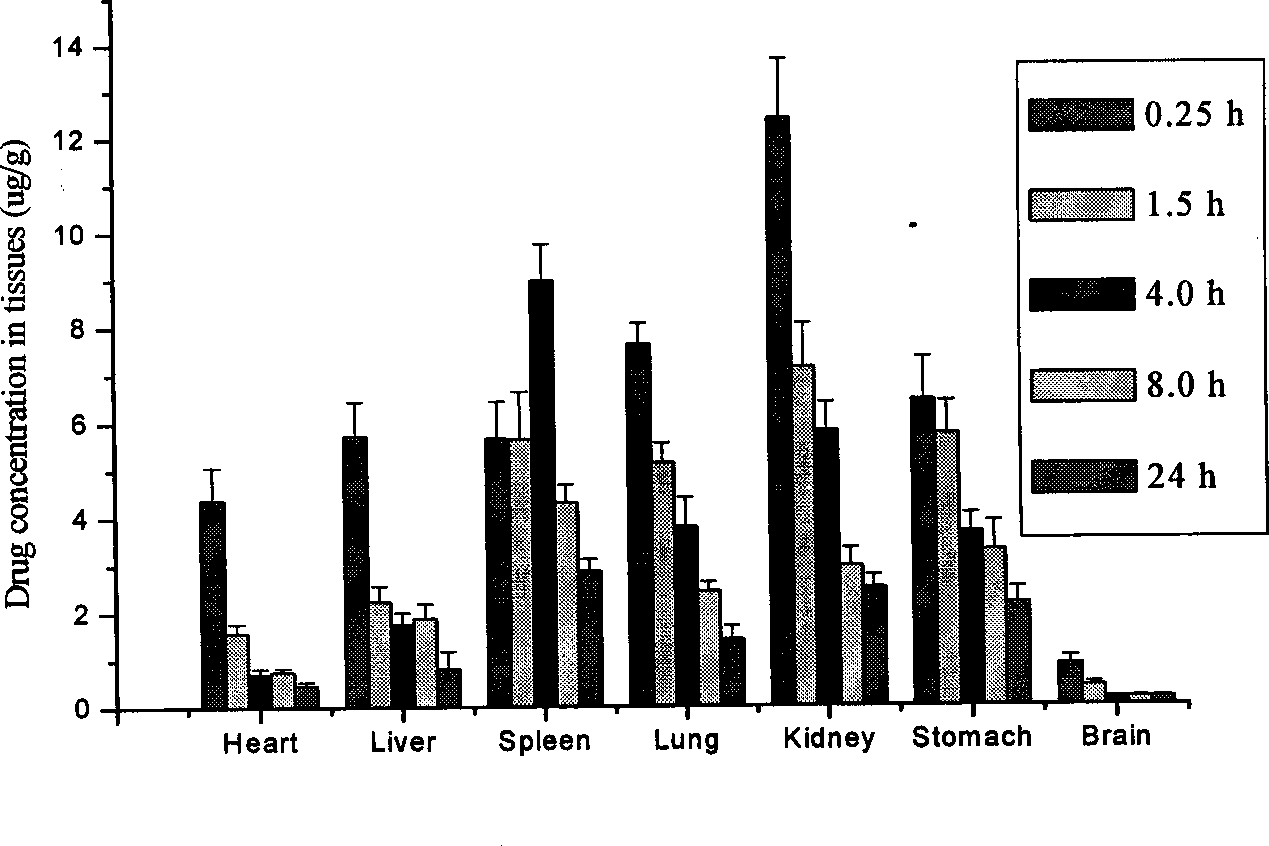
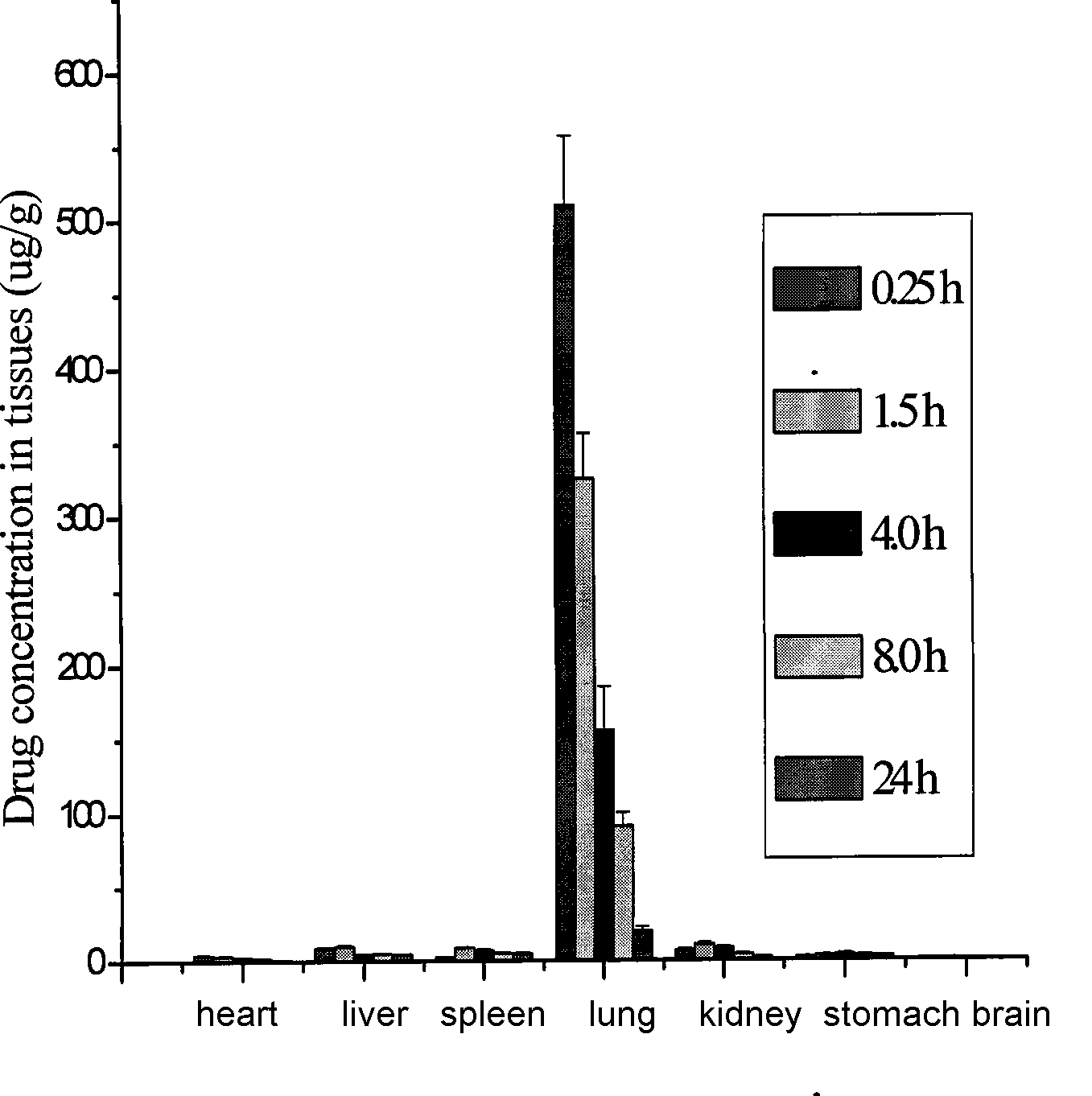
Lung-targeted medicine carrying precursor liposome for injection and method of use thereof
A technology for proliposomes and injections, applied in liposome delivery, pharmaceutical formulations, drug combinations, etc., can solve the problems of high cost, low encapsulation rate of water-soluble drugs, and unguaranteed safety of intravenous administration and other issues to achieve the effect of stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Example 1: The lung-targeted drug-loaded proliposome for injection is prepared by the following steps:
[0094] a. Take the following materials in the following proportions by weight:
[0095] Docetaxel 4mg
[0096] Dicetyl Phospholipids 10mg
[0097] Hydrogenated Soy Lecithin 20mg
[0098] Cholesterol 15mg
[0099] Tween-80 20mg
[0100] Citric acid 90mg
[0101] And the above-mentioned substances were dissolved in absolute ethanol to obtain a 10% (mg / ml) solution A;
[0102] b. Add 0.5% of its volume of activated carbon for injection into solution A, stir and let it stand, and filter through a microporous membrane with a pore size of 0.22 μm to obtain sterile and pyrogen-free solution B;
[0103] c. Under sterile conditions, add 3 mg of polyvinylpyrrolidone solution B, and then remove the organic solvent under reduced pressure under general mechanical stirring to obtain solid granular drug-loaded proliposomes;
[0104] d. Determining the main drug content of th...
Embodiment 2
[0107] Example 2: The lung-targeted docetaxel proliposome for injection is prepared by the steps in the following sequence:
[0108] a. Take the following materials in the following proportions by weight:
[0109] Docetaxel 0.001mg
[0110] Soy Lecithin 15mg
[0111] Phosphatidylserine 8mg
[0112] Cholesterol 10mg
[0113] Tween-80 20mg
[0114] Tartaric acid 60mg
[0115] Citric acid 10mg
[0116] And the above-mentioned substances were dissolved in absolute ethanol to obtain a 5% (mg / ml) solution A;
[0117] b. Add 0.1% of its volume of activated carbon for injection to solution A, stir and let it stand, and filter with a microporous membrane with a pore size of 0.15 μm to obtain sterile and pyrogen-free solution B;
[0118] c. Under sterile conditions, add 2 mg of vitamin E, 5 mg of mannitol and 3 mg of polyvinyl rolidone into solution B, and then remove the organic solvent under reduced pressure with general mechanical stirring to obtain solid granular polyvinyl ro...
Embodiment 3
[0122] Example 3: The lung-targeted docetaxel proliposome for injection is prepared by the steps in the following sequence:
[0123] a. Take the following materials in the following proportions by weight:
[0124] Docetaxel 8mg
[0125] Lecithin 30mg
[0126] Beta-Sitosterol 15mg
[0127] Tween-80 15mg
[0128] Tartaric acid 25mg
[0129] And the above-mentioned substances were dissolved in a mixed solvent of absolute ethanol and absolute ether, the volume ratio of absolute ethanol and absolute ether in the mixed solvent was 7:3, and a solution A of 30% (mg / ml) was obtained;
[0130] b. Add 0.3% of its volume of activated carbon for injection into solution A, stir and let it stand, and filter with a microporous membrane with a pore size of 0.18 μm to obtain sterile and pyrogen-free solution B;
[0131] c. Under sterile conditions, 5 mg of vitamin E was added to solution B, and then the organic solvent was removed by a closed-loop spray drying device to obtain powdered docet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Surface charge | aaaaa | aaaaa |
| Surface charge | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com