Human streptococcus mutans genetic engineering vaccine for decayed tooth and preparation method thereof
A Streptococcus mutans and genetic engineering technology, applied in the field of human Streptococcus mutans genetically engineered dental caries vaccine and its preparation, to achieve high-efficiency immune response, good immune protection, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
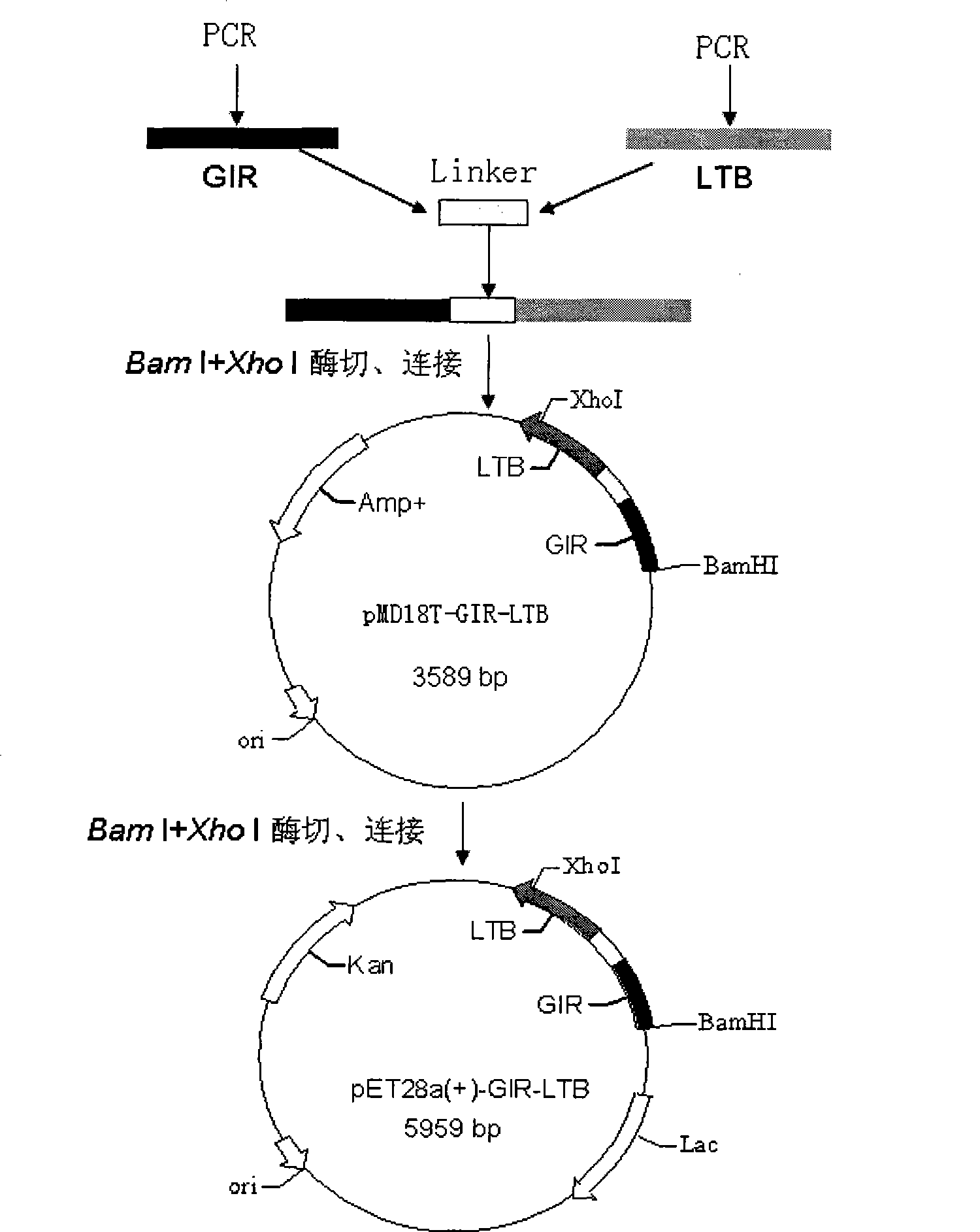
[0105] Example 1 Construction of expression vector of fusion gene GIR-LTB
[0106] 1. Primer design and PCR amplification
[0107] 1) The primers were designed as follows: According to the gene encoding GIR and the heat-labile enterotoxin B subunit LTB gene of S. Mutans (UA159 standard strain) found in GenBank, the software Primer Premier 5.0 was used to design and analyze primers. Design the linker (frame region) sequence at the 5' end of the upstream gene (GIR) and introduce the BamHI site, introduce the linker (frame region) sequence at the 3' end, and introduce the linker sequence at the 5' end of the downstream gene LTB (framed part), the XhoI site was introduced into the 3' end, and the GIR gene and the LTB gene were connected by the overlap extension PCR method. Primers were synthesized by Shanghai Yingjun Company.
[0108] GIR: Upstream primer P1: SEQ ID NO: 5
[0109] 5'- CAAGCACAAGTTAAT-3' (BamHI)
[0110] Downstream primer P2: SEQ ID NO: 6
[0111] 5'- A...
Embodiment 2
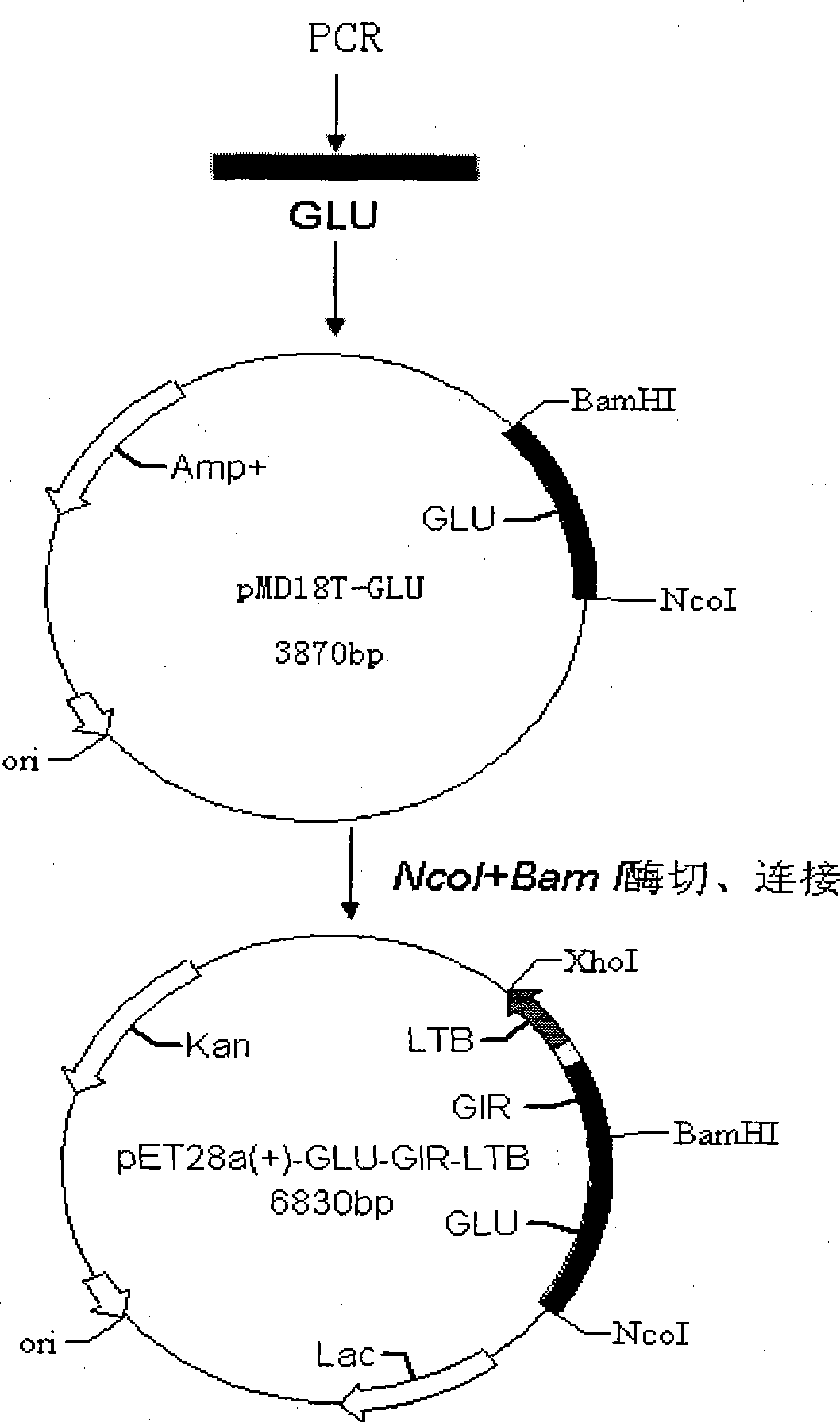
[0127] Example 2 Construction and expression of GLU-GIR-LTB fusion gene expression vector and recombinant expression engineering bacteria
[0128] 1. Primer design and PCR amplification
[0129] 1) The primers were designed as follows: according to the gene encoding GLU of S. Mutans (UA159 standard strain) found from GenBank, the primers were designed and analyzed using the software Primer Premier 5.0. The linker (frame region) sequence is designed at the 3' end of the GLU coding gene, and the upstream and downstream primers are respectively introduced into the NcoI and BamHI sites, and the sticky ends digested with BamHI can be combined with the fusion gene (GIR-LTB) Connect correctly. Primers were synthesized by Shanghai Yingjun Company.
[0130] GLU: upstream primer P1: SEQ ID NO: 9
[0131] 5'CCATGGATGAAATGGGCTATCAAGC-3'
[0132] Downstream primer P2: SEQ ID NO: 10
[0133] 5'- CCGAACTCGTTTCCAG-3'
[0134] 2) PCR amplification of the target gene
[0135] Strept...
Embodiment 3
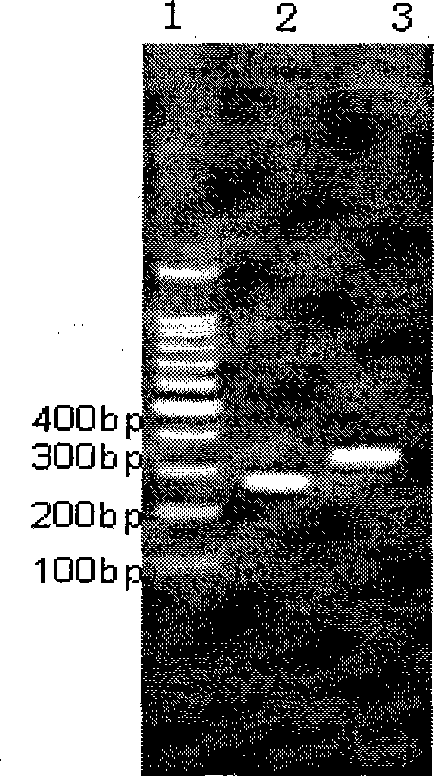
[0148] Example 3 Preliminary Study on Immunogenicity of Fusion Protein
[0149] 1. Immunization of Mice
[0150] Purified target protein GLU-GIR-LTB was used to immunize 4-5 week-old Balb / c mice, 100ug / mouse / time, 100μL of antigen was mixed with the same amount of Freund's complete adjuvant, and injected subcutaneously into the abdomen and groin of mice. The immunization program is: 0, 1, 2 weeks, a total of 3 times, Freund's complete adjuvant is added for the 1st and 2nd time, no adjuvant is added for the 3rd time, inoculated in the abdomen and groin subcutaneous of mice, and the amount of antigen and adjuvant injected is about 0.2ml, once a week, 6 days after the third immunization, the tail was docked to collect blood, and ELISA was used to detect the change of serum specific antibody titer.
[0151] 2. Detection of specific immune response.
[0152] (1) Preparation of ELISA antigen-coated plates:
[0153] Dilute GLU antigen to 5 μg / ml with coating solution, coat ELIS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com