Method for preparing granisetron hydrochloride from high tropine alkanamine
A technology for granisetron hydrochloride and tropane amine salt, which is applied in the field of preparing granisetron hydrochloride from high tropane amine salt, can solve the problems of inconvenient storage and transportation, influence, yellowing due to light, etc. Effects of transport and storage, mild conditions, low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
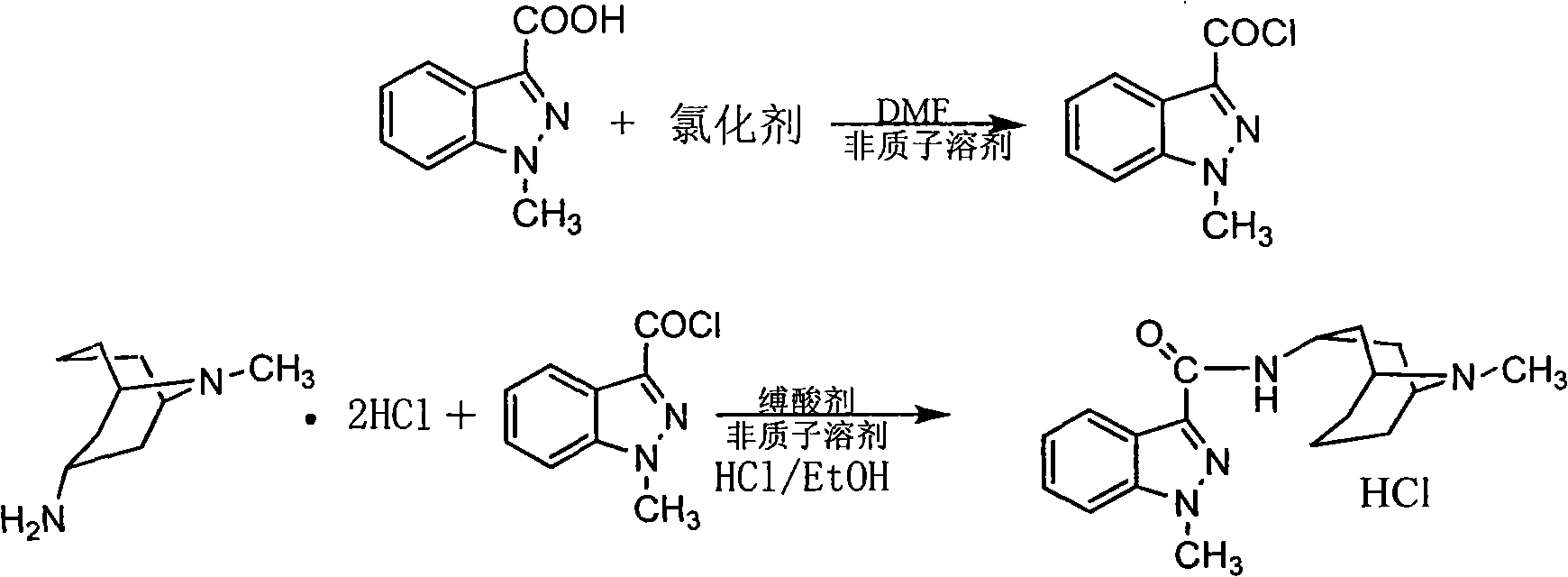
[0031] Add 3000ml of dichloromethane, 71.1g of 1-methylindazole-3-carboxylic acid, and 34.6ml of oxalyl chloride into a 5000ml three-necked flask, and stir. 7.1ml of N,N-dimethylformamide was added dropwise, and the reaction was stirred for 4 hours. Evaporate dichloromethane, re-add 3000ml dichloromethane to dissolve, add 130g homotropaneamine hydrochloride, 180ml triethylamine, and stir until the next day. The solution is yellow and transparent. Stirring was stopped and washed three times with saturated sodium bicarbonate solution. Add anhydrous potassium carbonate, stir and dry. Filter and recover dichloromethane under normal pressure, evaporate the solvent under reduced pressure, add 1000ml of absolute ethanol, and adjust the pH value to 2-3 with ethanol solution of hydrogen chloride. Add seed crystals. Filter to get crude product. Recrystallization from water and ethanol gave 110.7 g of the final product. Yield: 87.7%. Melting point: 290.2-291.1°C.
[0032] This ex...
Embodiment 2
[0034] Add 6000ml of chloroform, 142.2g of 1-methylindazole-3-carboxylic acid, and 100ml of thionyl chloride into a 10000ml three-necked flask, and stir. 14.2ml of N,N-dimethylformamide was added dropwise, and the reaction was stirred for 4 hours. Evaporate chloroform, add 3000ml chloroform again to dissolve, add 188g high tropine amine sulfate, 130ml dimethylamine, stir until the next day. The solution is yellow and transparent. Stirring was stopped and washed three times with saturated sodium bicarbonate solution. Extract with water and add 130ml12N hydrochloric acid. The measured pH value is 2-3. Water and 20ml hydrochloric acid were added to extract twice. The aqueous phases were combined and washed once with chloroform. Extract with chloroform plus 10N sodium hydroxide solution, the pH value is 10-11. Extract twice with chloroform, and combine the chloroform layers. Wash once with saturated sodium bicarbonate solution. Wash once with saturated saline. Add anhydro...
Embodiment 3
[0036] Add 6000ml of ethyl acetate, 142.2g of 1-methylindazole-3-carboxylic acid, and 50ml of phosphorus oxychloride into a 10000ml three-necked flask, and stir. 14.2ml of N,N-dimethylformamide was added dropwise, and the reaction was stirred for 4 hours. Evaporate ethyl acetate, add 3000ml ethyl acetate again to dissolve, add 260g high tropine amine dihydrogen sulfate, 240ml diethylamine, stir until the next day. The solution is yellow and transparent. Stop stirring, wash once with water, and wash twice with saturated sodium bicarbonate solution. Extract with water and add 130ml12N hydrochloric acid. The measured pH value is 2-3. Water and 20ml hydrochloric acid were added to extract twice. The combined aqueous phases were washed once with ethyl acetate. Extract with ethyl acetate plus 10N sodium hydroxide solution, the pH value is 10-11. It was extracted twice with ethyl acetate, and the ethyl acetate layers were combined. Wash once with saturated sodium bicarbonate s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com