Method for obtaining transgenic chicken by lentivirus vector mediated in vivo transfection of spermatogonial stem cell
A technology of lentiviral vectors and spermatogonial stem cells, applied in the field of obtaining transgenic chickens, can solve the problems of difficult acquisition and implantation of poultry eggs and fertilized eggs, few transgenic individuals, and limited acquisition methods, and achieve high production practical value and genome structure Improved, easy-to-operate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Construction of a lentiviral vector plasmid expressing green fluorescent protein.
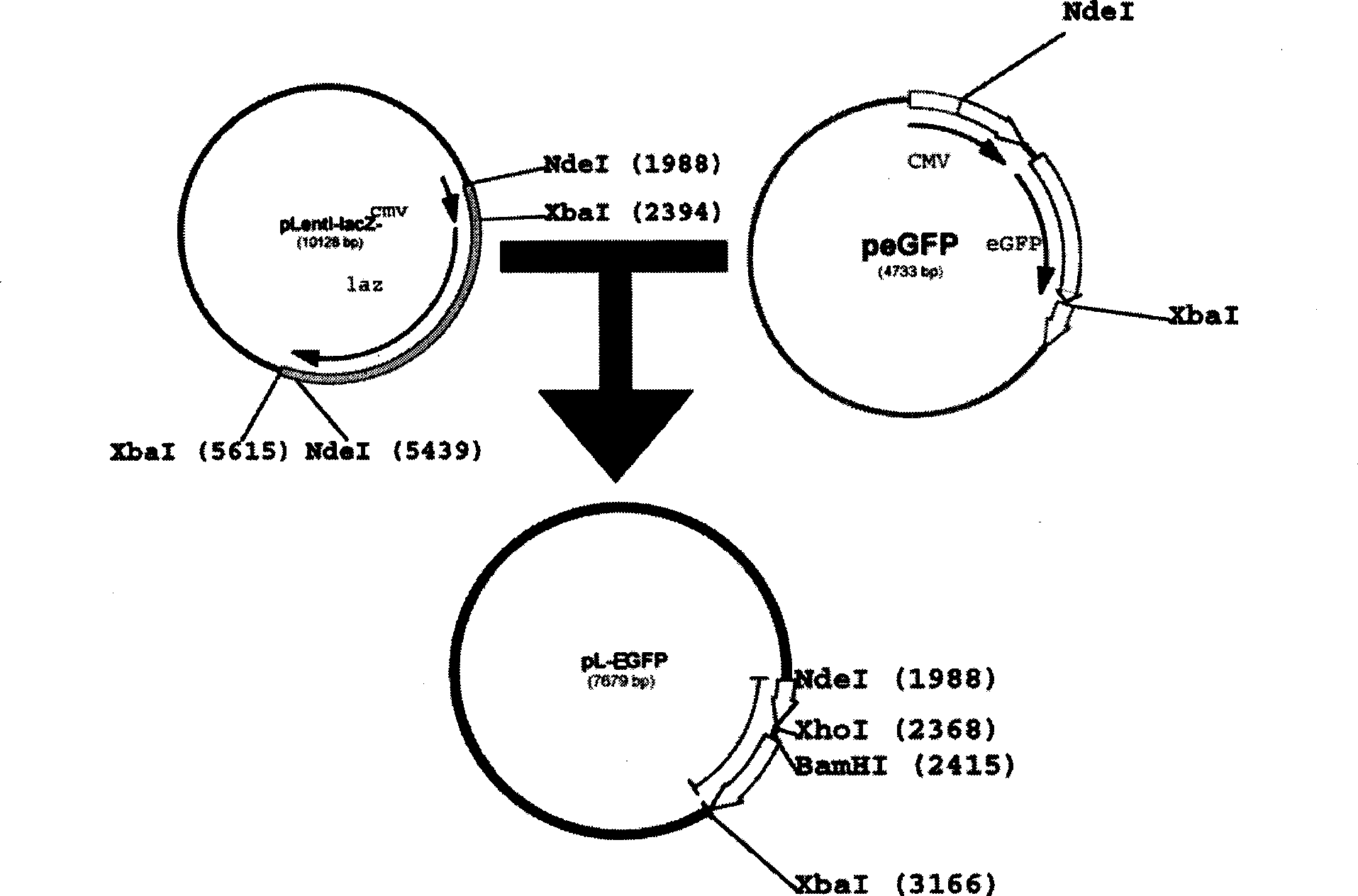
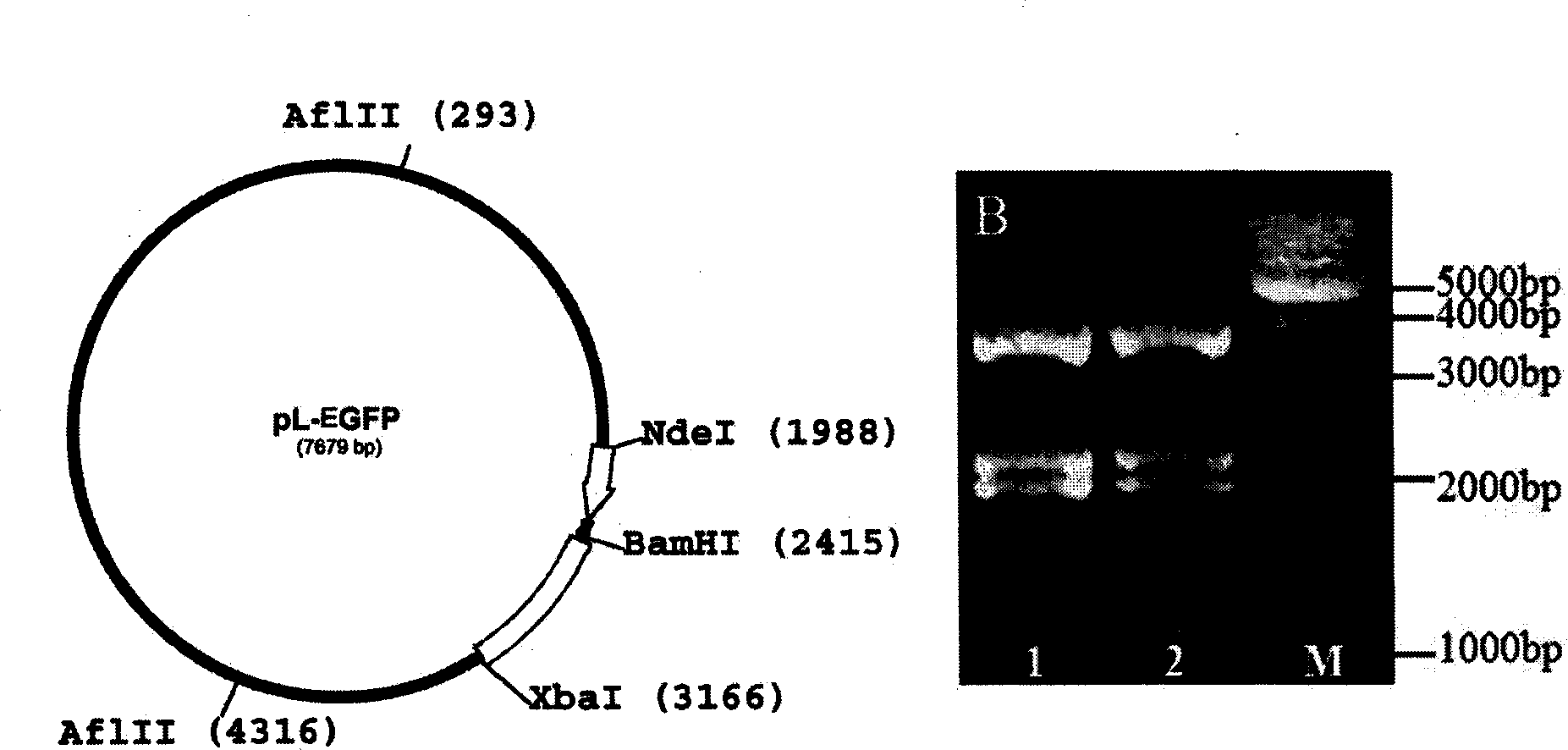
[0051] The enhanced green fluorescent protein gene (EGFP) comes from the peGFP plasmid (GeneBank Accession No: U55762, Clontech Company), which is obtained by separating and purifying the peGFP plasmid after Nde I and Xba I (NEB Company) double enzyme digestion and agarose gel electrophoresis . The pL-lacZ plasmid (Invitrogen) was digested with the same enzyme to obtain a DNA fragment containing the lentiviral transfer vector sequence, and the vector was dephosphorylated (NEB) with thermozyme phosphatase, separated and purified by agarose gel electrophoresis, and then purified with a quick ligation kit (LigaFastTM Rapid DNA Ligation System, Promega) was linked with EGFP, and finally transfected with JM109 competent bacteria to amplify the plasmid, and the new plasmid was named pL-EGFP ( figure 1 ).
[0052] Analyzed by Gene Construction Kit 2.0 software, the pL-EGFP plasmid ...
Embodiment 2
[0053] Example 2: Construction of a lentiviral vector plasmid expressing human tissue plasminogen activator (tPA) green fluorescent protein fusion protein.
[0054] In order to insert tPA into the viral vector, primers were designed based on the tPA sequence to introduce Xho I and BamH I restriction sites before and after the tPA coding region. The forward primer introduced four site mutations before the start codon ATG, and the change of +3 position from A to G did not change the conserved sequence of Kozak transcription initiation and did not affect the transcription efficiency of tPA in eukaryotic cells. The reverse primer introduces three mutation sites before the stop codon, which will result in the deletion of the last three amino acids after digestion and fusion with the EGFP gene.
[0055] Primer 1: GCGGGAATT CTCGAG ATGGATGCAA is underlined to indicate the Xho I restriction site
[0056] Primer 2: GATTATCAC GGATCC ATGTTGTCAC is underlined to indicate the restricti...
Embodiment 3
[0060] Example 3: Production of virus.
[0061] 1) One day before transfection, digest 293FT cells with trypsin, and take about 6×10 6 Cells are planted at 75cm 2 Add about 10ml of complete medium to the culture bottle for culture.
[0062] 2) On the day of transfection, when the cells reach 90-95% confluence, remove the culture medium and add 6ml of 293FT medium without double antibodies.
[0063] 3) Prepare DNA-liposome transfection complex:
[0064] A: Take 11.25 μg of virus-assisted packaging plasmid mixture (invitrogen) and 3.75 μg of pL-EGFP or pL-tPAGFP plasmid, a total of 15 μg of DNA, and add it to 1.9mL 293FT serum-free medium without double antibodies, mix gently, and set aside.
[0065] B: Take another centrifuge tube and add 45 μL of liposome Lipofectamine TM 2000 (invitrogen) into 1.9mL 293FT serum-free and double-antibody-free medium, gently blow and aspirate to mix, and incubate at room temperature for 2-4min (no more than 5min).
[0066] Quickly mix with ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com