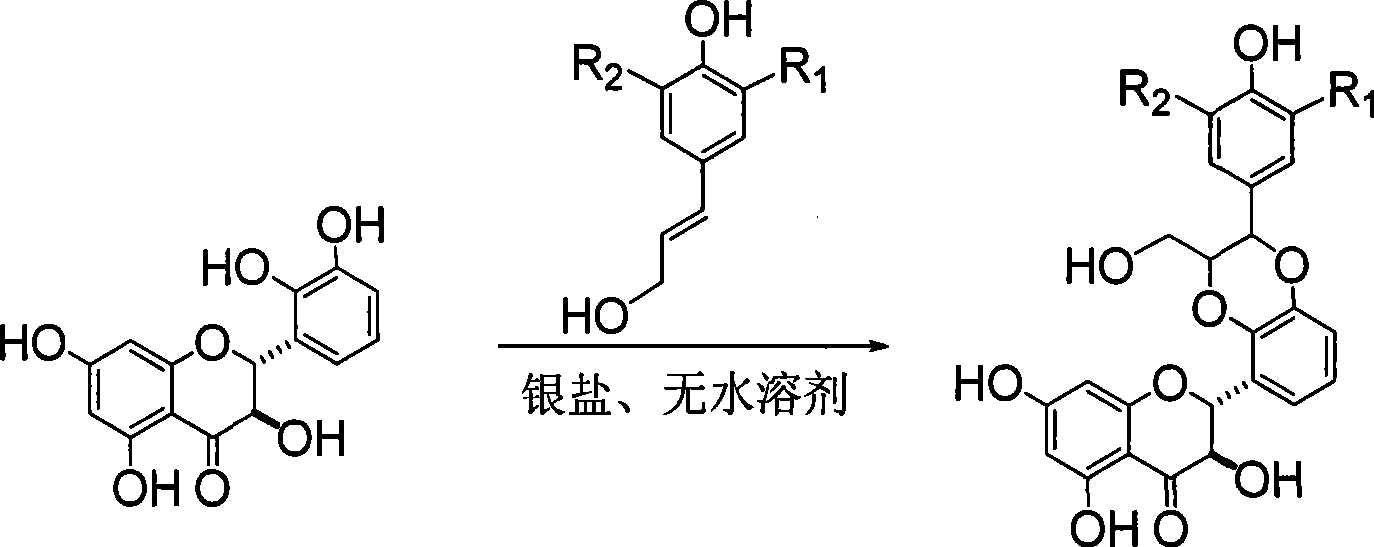
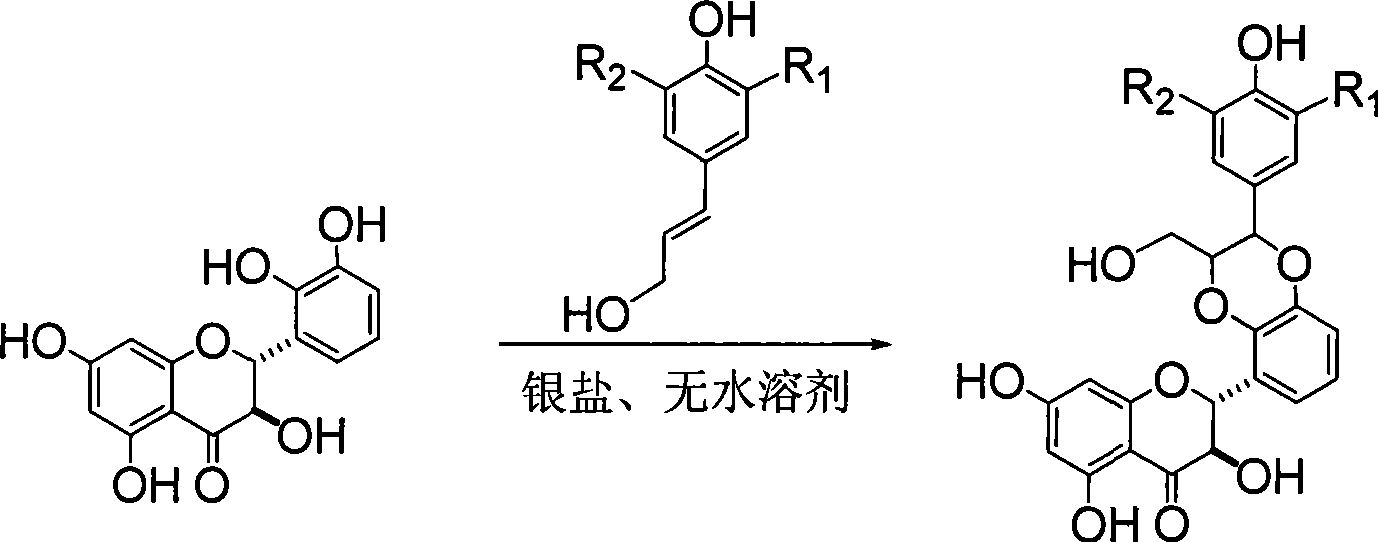
Xylogen like flavonoid compounds, method of preparing the same and pharmaceutical use
A compound and lignin technology, applied in the field of medicine, can solve the problems of water solubility and bioavailability limiting the drug market, and achieve the effects of inhibiting xanthine oxidase activity, low human toxicity, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
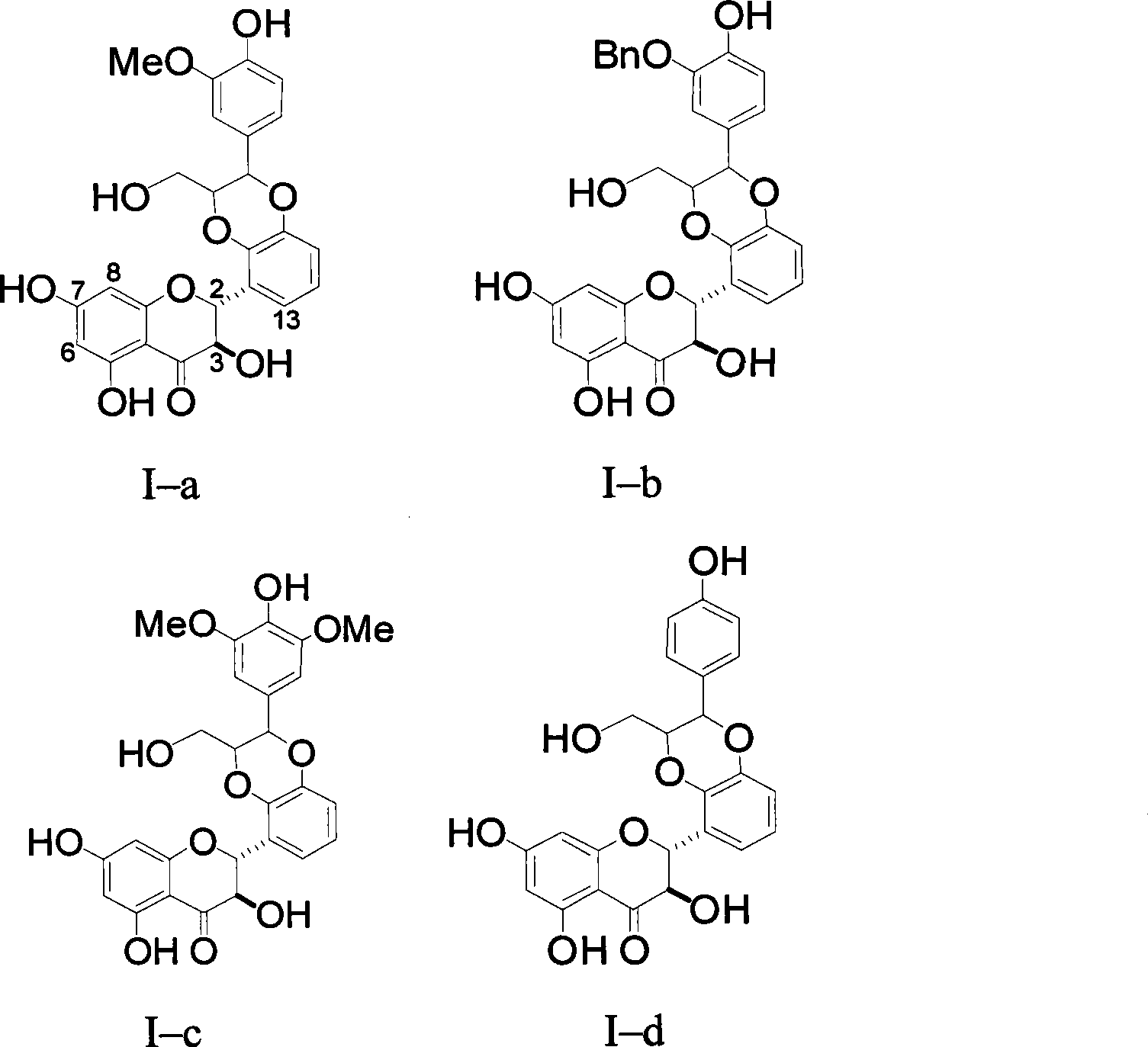
[0026] Example 1 : Compound I-a is (±)-2-[2,3-dihydro-2-(3-methoxy-4-hydroxyphenyl)-3-hydroxymethyl-1,4 benzodioxane- 5] Preparation of -2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one
[0027]
[0028] 1.1 Preparation of starting material A:
[0029] starting material A
[0030]Dissolve 4.8 g of 2,3-dihydroxybenzaldehyde in 30 ml of acetone, stir for 10 minutes, add 17.5 g of potassium carbonate, then dropwise add 6 ml of chloromethyl ether, heat to reflux for 1 hour, filter, and concentrate the filtrate to obtain 7.0 g of yellow oil , used directly in the next reaction.
[0031] 1.2 Preparation of starting material B:
[0032] starting material B
[0033] 2.6 grams of sodium hydride in 40 milliliters of DMF was cooled in an ice-water bath, and a mixed solution of 5.6 grams of 2,4,6-trihydroxyacetophenone in 60 milliliters of benzene and 7.0 milliliters of DMF was added dropwise under nitrogen protection, and cooled in an ice bath 9.0 ml of chloromethyl ether ...
Embodiment 2
[0045] Example 2 : Compound I-b is (±)-2-[2,3-dihydro-2-(3-benzyloxy-4-hydroxyphenyl)-3-hydroxymethyl-1,4 benzodioxane- 5] Preparation of -2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one
[0046]
[0047] According to the synthesis method described in Example 1, from (±)-2-(2,3-dihydroxyphenyl) 2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyridine The coupling reaction between pyran-4-one and 3-benzyloxy-4-hydroxycinnamyl alcohol finally obtained 36 mg of compound I-b. R f (chloroform / methanol / ethyl acetate / acetone / acetic acid=11:0.5:1:1:0.1): 0.29; electrospray mass spectrometry ESI-MS: 557 (M-1) + .
Embodiment 3
[0048] Example 3 : Compound I-c is (±)-2-[2,3-dihydro-2-(3,5-dimethoxy-4-hydroxyphenyl)-3-hydroxymethyl-1,4 benzodiox Preparation of Hexacyclo-5]-2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyran-4-one
[0049]
[0050] According to the synthesis method described in Example 1, from (±)-2-(2,3-dihydroxyphenyl) 2,3-dihydro-3,5,7-trihydroxy-4H-1-benzopyridine The coupling reaction between pyran-4-one and 3,5-dimethoxy-4-hydroxycinnamyl alcohol finally obtained 26 mg of compound I-c. R f (chloroform / methanol / ethyl acetate / acetone / acetic acid=11:0.5:1:1:0.1): 0.24; electrospray mass spectrometry ESI-MS: 511 (M-1) + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Half inhibitory concentration | aaaaa | aaaaa |
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com