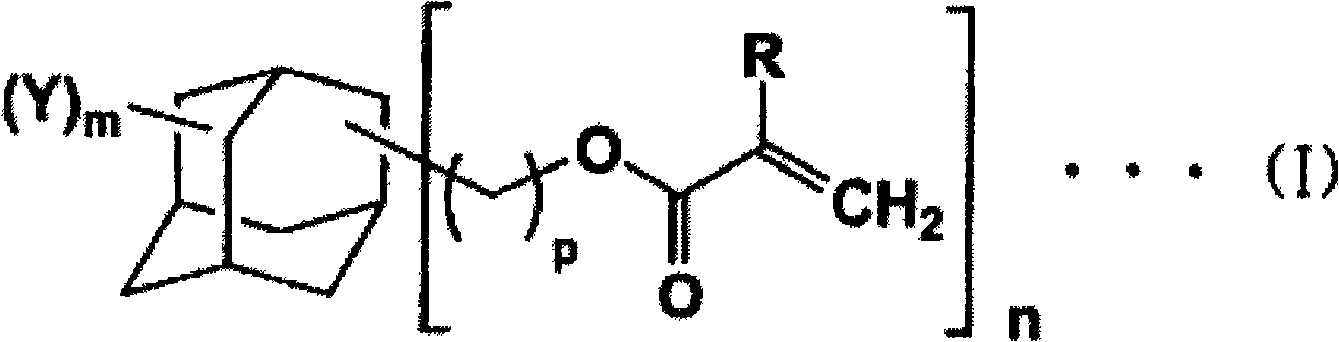
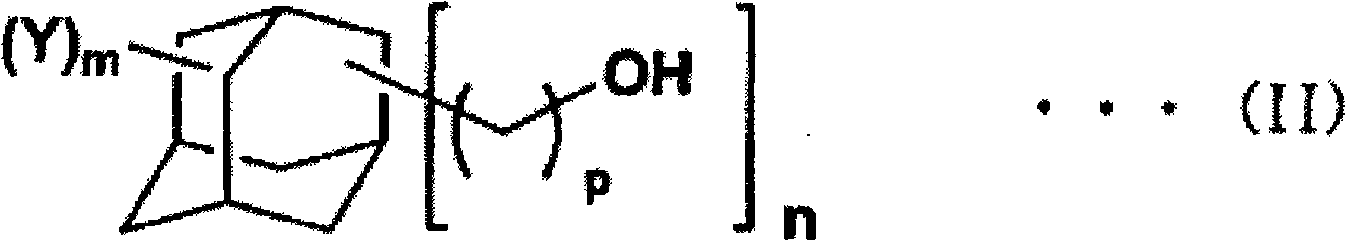
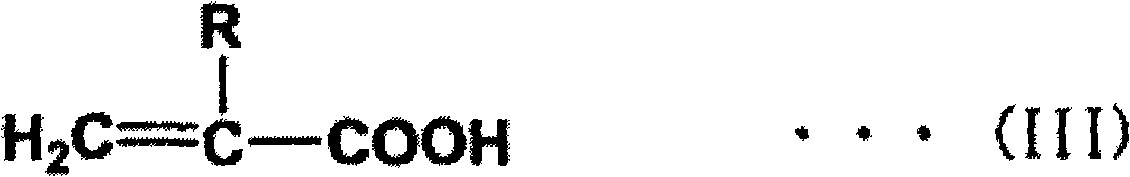Adamantane derivative, process for production thereof, resin composition, and cured product of the resin composition
A technology of derivatives and adamantane, which is applied in the field of adamantane derivatives, its manufacture, resin composition and its cured products, can solve the problems of unsatisfactory heat resistance and mechanical strength, and achieve curing shrinkage or linear expansion coefficient Low heat resistance and excellent mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1 "Synthesis of Adamantane-1,3,5-trimethanol triacrylate"
[0112] Add 50g adamantane-1,3,5- Trimethanol (0.22 mol), 55.7 g of acrylic acid (0.77 mol), 500 mL of toluene, 1.11 g of 98% by mass sulfuric acid and 0.06 g of p-methoxyphenol were immersed in an oil bath at 130° C. and heated to reflux for 3 hours. At this time, water gradually produced as the reaction proceeds is removed from the reaction system while the reaction proceeds. Then, the reaction solution was cooled to room temperature and transferred into a separatory funnel. Subsequently, 250 mL of 5% by mass sodium chloride aqueous solution was added for washing, and then the organic layer was washed with 250 mL of 3% by mass sodium phosphate aqueous solution, and then further washed with 250 mL of 5% by mass sodium chloride aqueous solution. Next, after dehydration with anhydrous magnesium sulfate, magnesium sulfate was removed by filtration. The filtrate was evaporated to remove the solvent to ob...
Embodiment 2
[0117] In 10 g of adamantane-1,3,5-trimethanol triacrylate obtained in Example 1, 0.1 g of benzoin isobutyl ether was added as a photopolymerization initiator, mixed thoroughly and vacuum degassed to obtain a resin composition. Pour the resin composition into a glass tank, and use a mercury lamp at 3000mJ / cm 2 Under the condition of irradiation, a cured product with a thickness of 1mm was obtained. The physical property values of the obtained cured product are shown in Table 1.
Embodiment 3
[0119] In the mixture of 7g of adamantane-1,3,5-trimethanol triacrylate and 3g of adamantane-1,3-dimethanol diacrylate obtained in Example 1, 0.1g of benzoin isobutyl ether was added as a light The polymerization initiator is fully mixed and then vacuum degassed to obtain a resin composition. Pour the resin composition into a glass tank, and use a mercury lamp at 3000mJ / cm 2 Under the condition of irradiation, a cured product with a thickness of 1mm was obtained. The physical property values of the obtained cured product are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com