Application of hypnopyrine to the preparation of 2-type antidiabetic drugs
A technology of type 2 diabetes and Hypno, applied in the field of biomedicine, can solve the problems of poor selection specificity, reduced druggability, poor chemical and biological stability, etc., and achieve novel action targets, long-term use price, and good prevention and treatment efficacy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
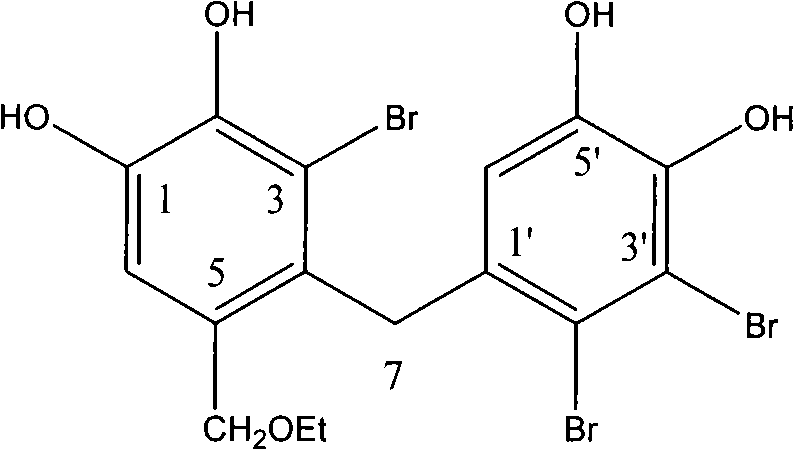
[0019] Example 1 Preparation and structure identification of "Hipnuo"
[0020] Turpentine algae (dry weight 20kg) was crushed and extracted with 100L of 95% ethanol, and the extract was concentrated under reduced pressure; the concentrate was suspended in 10L of distilled water, extracted with an equal volume of ethyl acetate, and concentrated under reduced pressure. The ethyl acetate extraction part was subjected to silica gel column chromatography, and the gradient elution was carried out with petroleum ether-acetone (100:0~1:1) until the volume ratio of the two was 1:1 (petroleum ether in the mixed eluent of petroleum ether and acetone) The volume concentration of chloroform changes from 100%-50% along the gradient), and chloroform-methanol (100:0~0~100) gradient elution is used instead (the volume concentration of chloroform in the chloroform-methanol mixed eluent changes from 100% along the gradient to -0% change), finally eluted with 95% ethanol, checked by thin-layer ch...
Embodiment 2
[0025] Example 2 Determination of protein tyrosine phospholipase 1B inhibitory activity
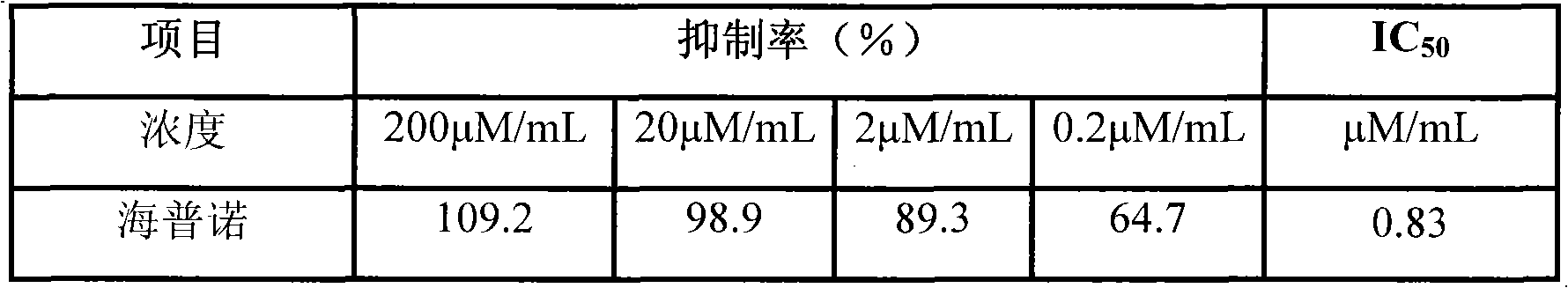
[0026] The test compound (3-bromo-4-[2',3'-dibromo-4',5'-dihydroxyphenyl]methyl-5-(ethoxy)-1,2-diphenol) Prepare the test solution with different concentrations in DMSO, take 2 μL of the test solution and add it to the standard bioassay system (50mM Tris-HCl, pH 6.5, 2mM pNPP, 2% DMSO, 30nM hGST-PTP1B), negative control : DMSO, positive control: sodium orthovanadate (2μM), the reaction temperature is 30°C, the light absorption at the wavelength of 405nm is dynamically measured, and the time is 3min. The inhibition rate and IC of compound PTP1B enzyme activity are calculated according to the following formula 50 (LOGIT method). Inhibition rate=(experimental group A value-negative control group A value) / (control group A value-negative control group A)×100%, the results are shown in Table 1.
[0027] Table 1 Inhibition rate (%) and IC of protein tyrosine phospholipase 1B 50
[0028] Tabl...
Embodiment 3
[0031] Embodiment 3 acute toxicity observation test
[0032] 20 Kunming mice were randomly divided into 4 groups, 3 females and 2 males in each group, separated into cages, and adaptively fed for 3 days; fasted for 16 hours before administration on the fourth day, drinking water was not limited, and 5000mg / kg, 500mg / kg and 50mg / kg doses were intragastrically administered ig, observed for 24 hours, and the number of mice died was 0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com