Alpha-keto-leucine-calcium preparation method
A technology of calcium ketoleucine and calcium chloride, which is applied in the field of preparation of calcium α-ketoleucine, can solve problems such as unsuitability for industrial production, harsh reaction conditions, and difficult availability of raw materials, so as to improve equipment utilization, Easy to get raw materials and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
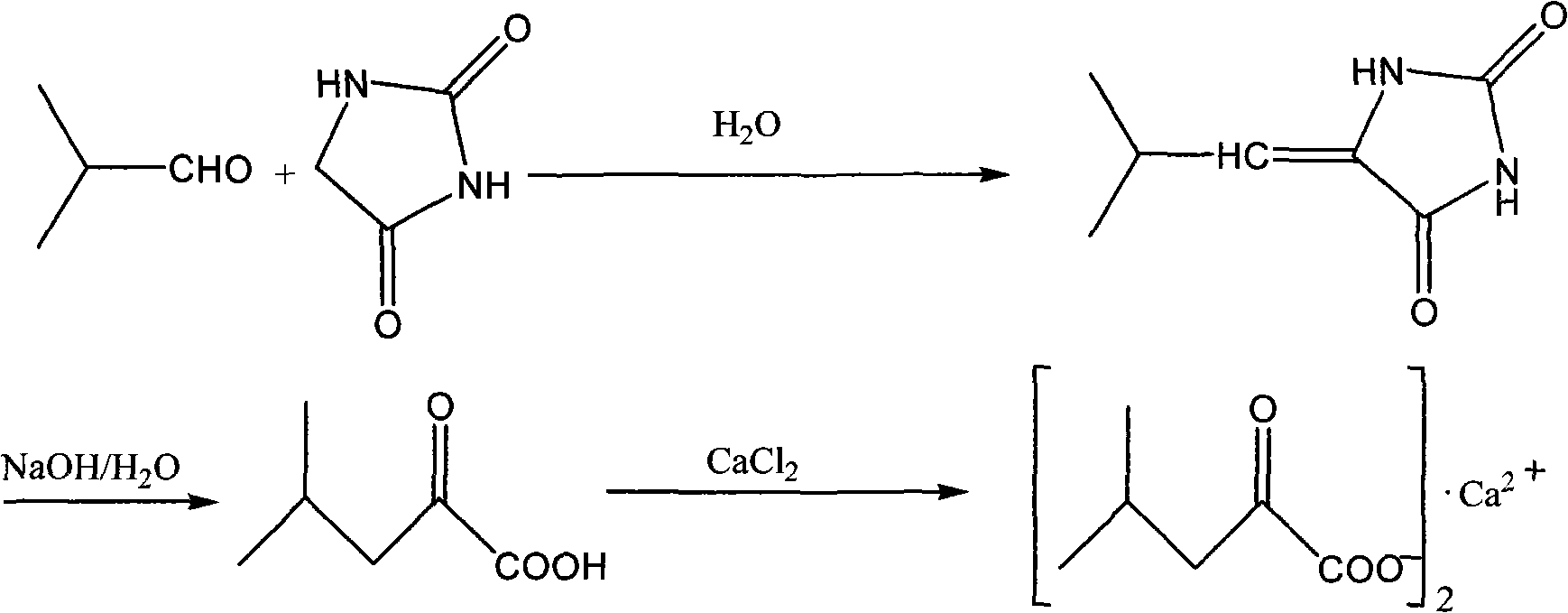
[0020] a. After mixing 100kg of hydantoin, 15kg of triethylamine and 560kg of water, stir and heat to 80°C, then add 110kg of isobutyraldehyde dropwise within 0.5 hours, then reflux for 7 hours and cool to Stir at 10-30°C to precipitate white solid isobutylidene hydantoin;
[0021] b. At 25°C, add dropwise 960kg of 40% sodium hydroxide (or potassium hydroxide) aqueous solution to the reaction solution at 25°C, stir and heat to 99~101°C for reflux reaction for 5 hours, then cool to room temperature with ice water, Use hydrochloric acid or sulfuric acid with a concentration of 30 to 50% by weight to adjust the pH to ≤2, extract three times with 500 kg of methyl tert-butyl ether solvent, combine the organic phases, and distill off the solvent under reduced pressure to obtain a purple-red liquid α-ketoleucine Crude.
[0022] c. After mixing 850kg of ethanol solution of calcium chloride and 50kg of triethylamine with a concentration of 8% by weight, the crude product of α-ketoleuc...
Embodiment 2
[0023] Embodiment 2: The difference between this embodiment and Example 1 is to replace 15kg triethylamine with 25kg ethanolamine; replace methyl tert-butyl ether solvent with 600kg ether; the methanol solution of calcium chloride (mass percentage concentration is 5%) ) and the diethanolamine of 40kg are mixed.
Embodiment 3
[0024] Embodiment 3: present embodiment and embodiment 1 difference are to replace 15kg triethylamine with 12kg sodium carbonate; Replace methyl tert-butyl ether solvent with 650kg isopropyl ether; A solution of isopropanol and 35kg of ethanolamine are mixed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com