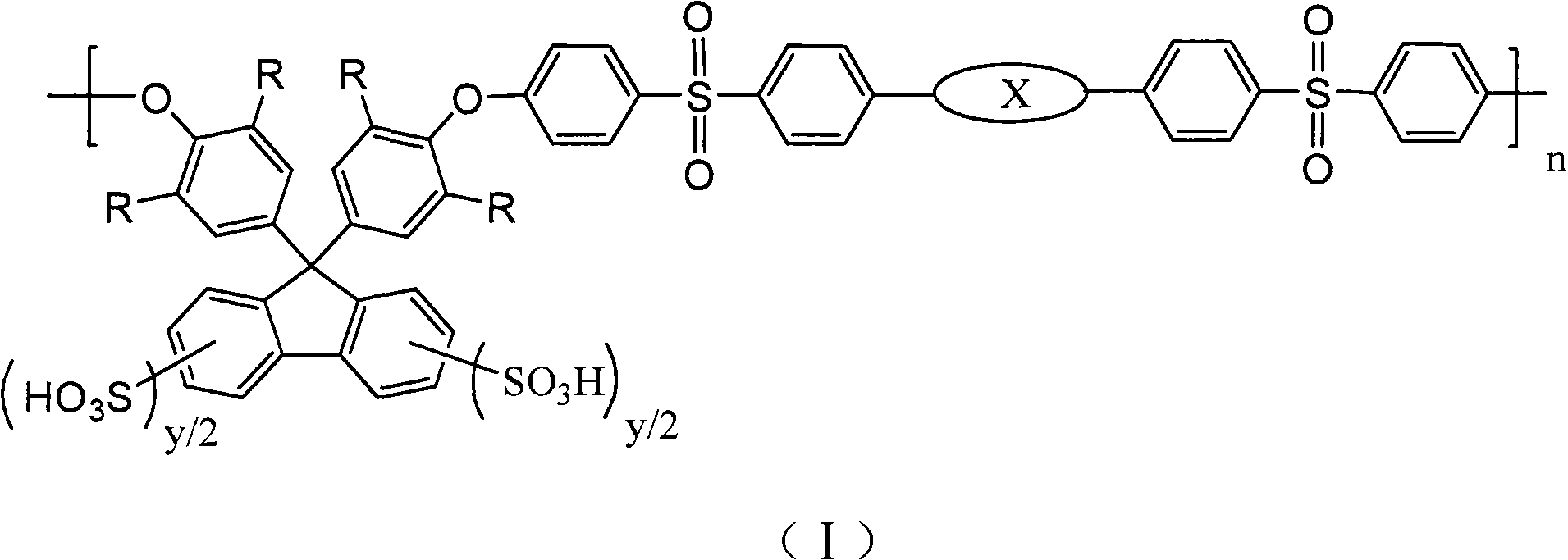
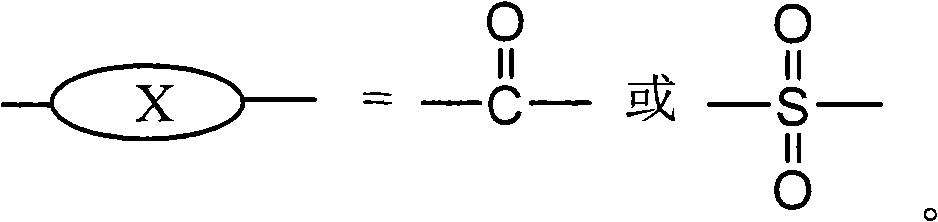
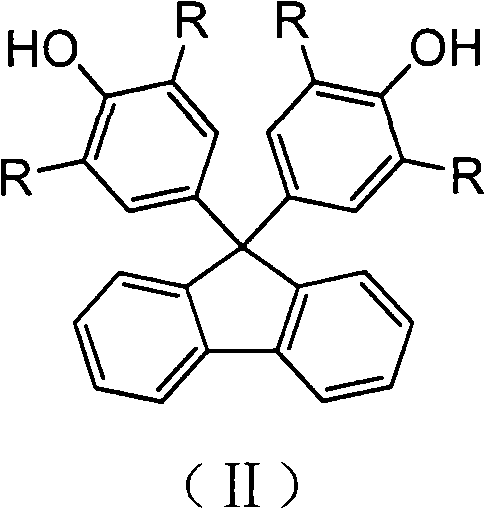
Sulfonated fluorene-containing polyether sulphone for proton exchange membrane of all-vanadium flow battery and preparation method and application thereof
An all-vanadium redox flow battery and proton exchange membrane technology, which is applied to fuel cell parts, battery pack parts, circuits, etc., can solve problems such as uncharacterized chemical stability, high vanadium ion permeability, and battery performance degradation , achieving high Coulombic efficiency and energy conversion efficiency, avoiding interaction, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Preparation of sulfonated fluorene-containing polyaryl ether sulfone:
[0040] At room temperature, add 0.5mmol of fluorene polyarylether sulfone 1aa into a 150mL single-necked flask, add 50mL of dichloromethane, and magnetically stir to dissolve it. Weigh 0.133mL (2mmol, 0.234g) of chlorosulfonic acid with a molar ratio of 1:6 (raw material ratio) to the chlorosulfonic acid molecule, add 10mL of dichloromethane to prepare a solution, and slowly add it dropwise with a constant pressure dropping funnel , react at room temperature for 6 hours, a brown precipitate is formed, pour out dichloromethane, wash the product 3 times with 10mL of cyclohexane, add 10mL of DMAc, stir for 1-2 hours to fully dissolve, add 20mL of 3wt% sodium hydroxide solution, Stir for 6 hours, add 100 mL of 5vol% hydrochloric acid solution, stir for 6 hours, transfer the product into a dialysis bag for dialysis until the water outside the bag is neutral, and then evaporate to dryness to obtain the su...
Embodiment 2~8
[0042] The sulfonation steps of embodiments 2, 3, 4, 5, 6, 7 and 8 are all the same as in embodiment 1, except that the raw material ratio or the chemical structure containing fluorene polyarylether sulfone, the sulfonation degree (y value) are different, as shown in Table 1:
[0043] Table 1 Results after sulfonation of fluorene-containing polyaryl ether sulfones with different molecular structures
[0044] Example
Embodiment 9
[0045] Embodiment 9 prepares single-component film
[0046] Take 1g of the sulfonated fluorene-containing polyarylether sulfone 2aa prepared in Example 1, add 20mL of DMAc, heat and stir to dissolve, filter, concentrate the filtrate to 10mL, cast it on a glass plate placed horizontally, and place it in a dust-free environment Dry at 80°C to obtain a single-component film 2-1.
[0047] The prepared film was Fenton's reagent (3wt.%H 2 o 2 +2ppm FeSO 4 ) to measure its anti-oxidation stability, and measure the time when the membrane begins to decompose into flocculent precipitates in a water bath shaker at 80°C.
[0048] The prepared membrane was acidified with 1M sulfuric acid at 80° C. for 24 hours, and then soaked in ultrapure water for 24 hours to wash away excess acid adsorbed in the membrane. The conductivity at room temperature was measured with an AC impedance meter.
[0049] The prepared film was VO 2+ The permeability is used to characterize the anti-vanadium ion ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com