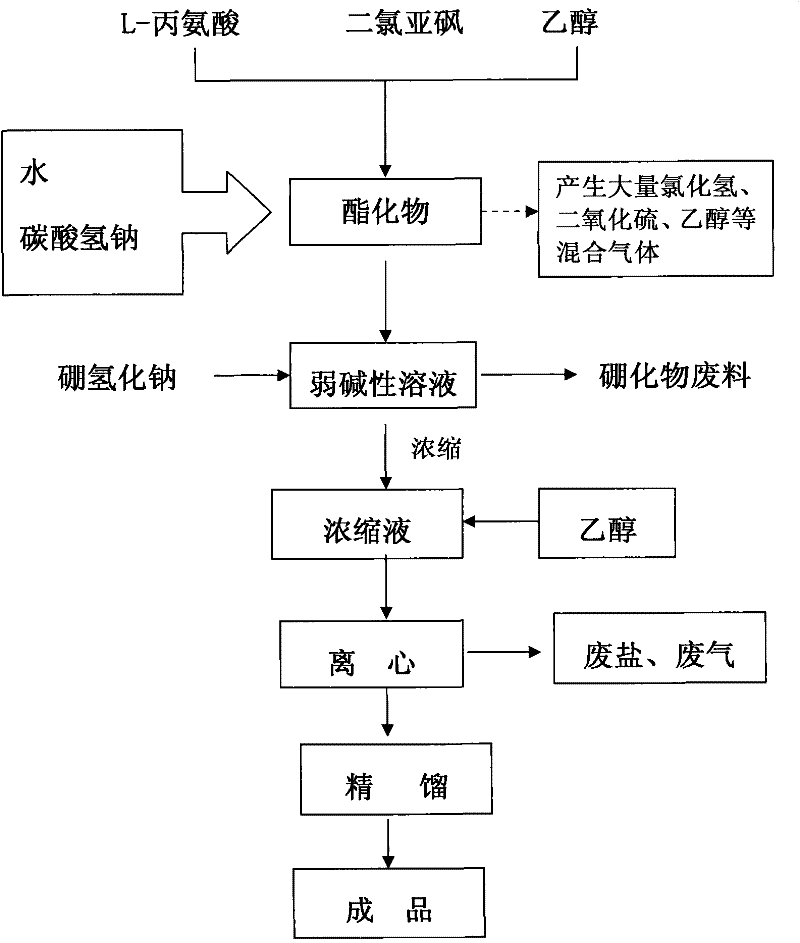
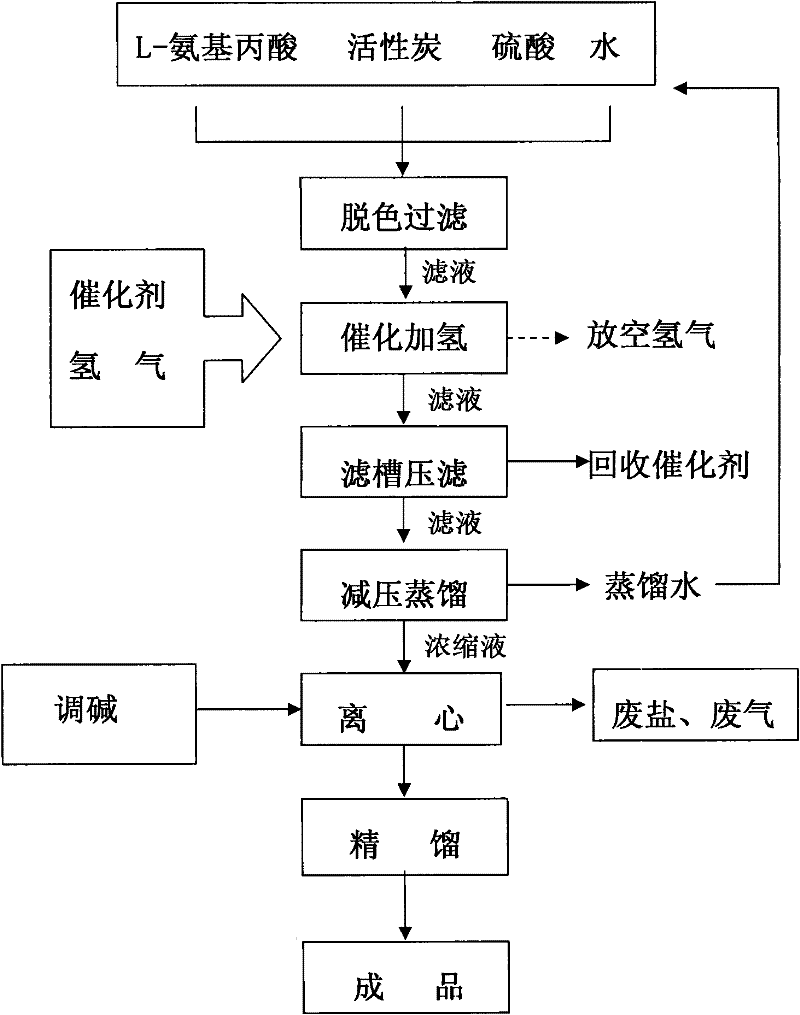
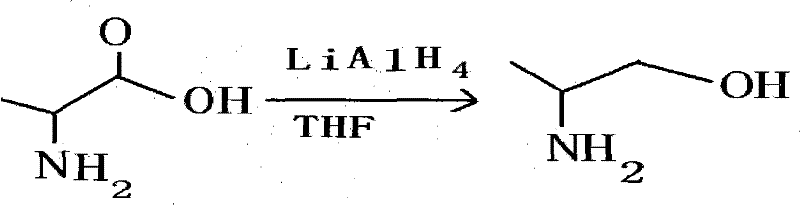Synthetic method of L-aminopropanol
A technology of aminopropanol and synthesis method, which is applied in the preparation of aminohydroxyl compounds, chemical instruments and methods, and the preparation of organic compounds, etc., which can solve the problems of prolonging the production cycle, flammability, and high risk, and achieve emission reduction of SOD and SO2 , reduce production costs, speed up the effect of response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of preparation method of L-aminopropanol of the present embodiment, it comprises the steps:
[0037] 1) Dissolve 50gL-alanine in 500ml of deionized water, then mix it with 50g of 85% sulfuric acid by mass;
[0038] 2) Add 250g of ruthenium carbon catalyst and pass in 5.6g (2.8mol) of hydrogen, control the reaction temperature at 88±2°C, pressurize at 8MPa, and stir at 500 rpm for 6 hours;
[0039] 3) After the reaction is finished, filter the filtrate, distill it under reduced pressure in a concentrated kettle, distill off the water, cool the concentrated solution, and filter to remove the insoluble substances therein after stirring evenly, and the obtained pure filtrate is rectified in a rectification tower, and then purified by high vacuum After rectification, L-aminopropanol with a purity of 99.5% is obtained, and the specific rotation reaches +17.70°.
[0040] Filter the filter cake of the ruthenium-containing carbon catalyst of gained, can return to step 2)...
Embodiment 2
[0043] A kind of preparation method of L-aminopropanol of the present embodiment, it comprises the steps:
[0044] 1) first dissolve 120gL-alanine in 850ml of deionized water, then mix it with 100g of 85% sulfuric acid by mass;
[0045] 2) Add 576g of ruthenium carbon catalyst and feed 12.32g (6.16mol) of hydrogen gas, control the reaction temperature to 82±2°C, pressurize at 9MPa, and stir at 600 rpm for 5 hours;
[0046]3) After the reaction is finished, filter the filtrate, distill it under reduced pressure in a concentrated kettle, distill off the water, cool the concentrated solution, and filter to remove the insoluble substances therein after stirring evenly, and the obtained pure filtrate is rectified in a rectification tower, and then purified by high vacuum After rectification, L-aminopropanol with a purity of 99.9% is obtained, and the specific rotation reaches +17.8°.
[0047] Filter the filter cake of the ruthenium-containing carbon catalyst of gained, can return ...
experiment example 1
[0049] The quality of the catalyst directly affects the effect of the hydrogenation reduction reaction, and the hydrogenation reaction mostly uses Pd / C, Pt / C, etc. as catalysts. When nickel catalyst is not effective for hydrogenation reduction, noble metal catalyst palladium, platinum, rhodium, etc. can be selected.
[0050] Preparation of Ruthenium Carbon Catalyst
[0051] Activated carbon-supported nail catalysts are widely used as catalysts for hydrogenation reactions due to their good hydrogenation performance. The methods for preparing ruthenium carbon catalysts include impregnation method, ion exchange method and liquid phase reduction method.
[0052] (a) Impregnation method The impregnation method is mainly to immerse the activated carbon and the parent in a certain solvent for a period of time, so that the parent compound can be more evenly adsorbed on the surface of the carrier, and then filter or evaporate the solvent at a certain temperature, dry, and then use Hi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
| specific rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com