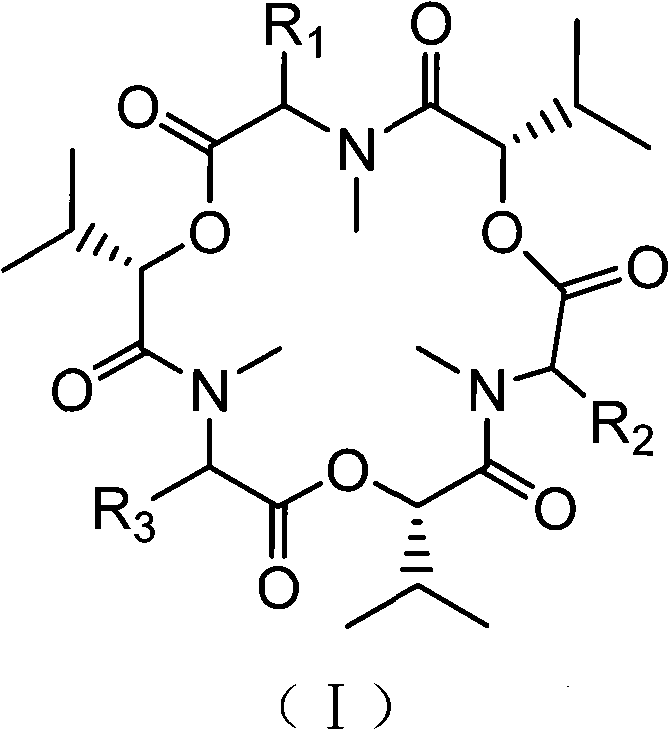
Application of enniatine compound for preparing anti-drug-resistant tubercle bacillus drugs
A technology of enfusaricin and compound, which is applied in the application field of enfusarium compound in the preparation of anti-drug-resistant tuberculosis drugs, can solve problems such as infection, multidrug resistance, etc., achieves easy purification, strong Activity against drug-resistant tuberculosis bacteria and its effect on large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
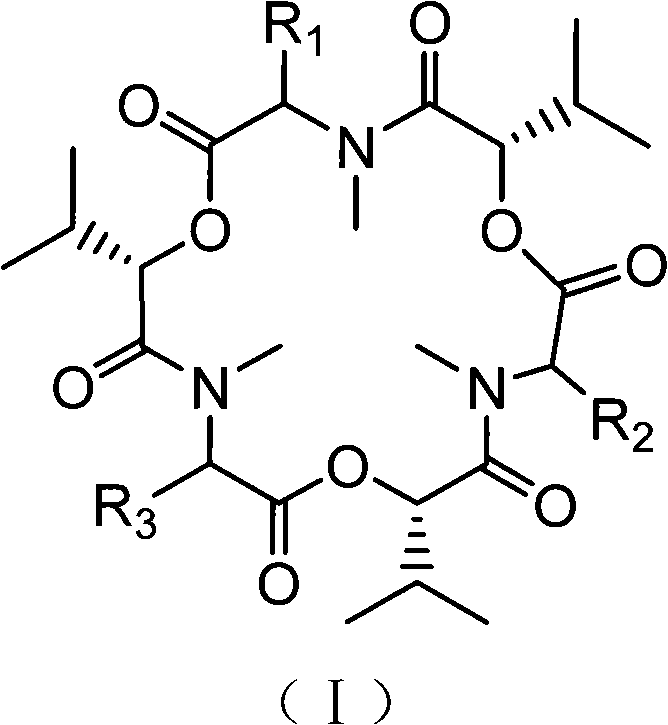
[0025] Preparation of enfusarin compounds shown in embodiment 1 formula (I)
[0026] In this example, GTY medium (containing 2.5 mass % glucose, 0.8 mass % peptone, 20 mass % artificial seawater with an initial value of pH 6) was used to carry out suspension fermentation culture of the endogenous fungus Fusarium sp. Static cultivation for 15 days, filtration, separation of bacteria and fermentation broth.
[0027] The above-mentioned fermentation broth was extracted with ethyl acetate, the extract was mixed with silica gel and packed into a column, and eluted with a gradient of petroleum ether-ethyl acetate-methanol. Obtain the crude product of the enfusarium compound mixture shown in formula (I) in the dehydration, and use ethyl acetate / cyclohexane (volume ratio is 1 / 1) mixed solvent isocratic elution, obtain enfusarium successively enfusarin G, enfusarin B, and enfusarin B 4 .
[0028] The above-mentioned bacteria were extracted with methanol, the extract was mixed with s...
Embodiment 2
[0029] Example 2 Anti-mycobacterium avium-intracellular activity test of enfusarin compounds
[0030] This embodiment adopts the solid medium dilution method to enfusarin G, enfusarin B, enfusarin B 4 The anti-Mycobacterium avium-intracellular activities of the four substances, Beauveria and Beauveria, were detected.
[0031] Take the culture of Mycobacterium avium intracellulare cultured on the slant, add it to 3ml Middlebrook7H9 broth medium, add a small amount of glass beads, tighten the cap of the test tube, vibrate vigorously on a vortex shaker, and compare with the standard McFarland turbidity Tube (MacFarland No.1) turbidity, that is to prepare a 1 mg / ml suspension of Mycobacterium avium intracellulare.
[0032] enfusarin G, enfusarin B, enfusarin B 4 and beauverum were formulated with DMSO (dimethyl sulfoxide) to prepare a high-concentration stock solution, and the stock solution was diluted to 120 μg / ml with 5% Tween-80 sterile ultrapure water. The sporin compounds...
Embodiment 3
[0035] Example 3 Anti-ISRE MTB strain activity test of enfusarin compounds against Mycobacterium tuberculosis clinical isolation
[0036] This embodiment adopts the solid medium dilution method to enfusarin G, enfusarin B, enfusarin B 4 Anti-tuberculosis Mycobacterium clinical isolates resistant to ISRE MTB strains (isoniazid, streptomycin, rifampicin, ethambutol-resistant Mycobacterium tuberculosis clinical isolates) activities were detected.
[0037] Scrape the culture of clinically isolated ISRE-resistant MTB strain of Mycobacterium tuberculosis from the inclined surface, add it to 3ml Middlebrook7H9 broth medium, add a small amount of glass beads, tighten the test tube cap, vibrate vigorously on a vortex shaker, and grind with standard wheat Turbidimetric tube (MacFarland No.1) was used for turbidimetry, that is, a 1 mg / ml bacterial suspension was prepared. This operation is performed in a biosafety level 3 laboratory.
[0038] enfusarin G, enfusarin B, enfusarin B 4 an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Minimum inhibitory concentration | aaaaa | aaaaa |
| Minimum inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com