Alkyl substituted-S,S-dioxo-dibenzothiophene monomer, preparation method and polymer thereof
A dibenzothiophene and alkyl technology, applied in the field of photoelectric materials, can solve the problems of poor solubility and limited application, and achieve the effects of low price, improved solubility, increased band gap and triplet energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of 2,8-dioctyl-3,7-dibromo-S, S-dioxo-dibenzothiophene
[0055] 1)
[0056]
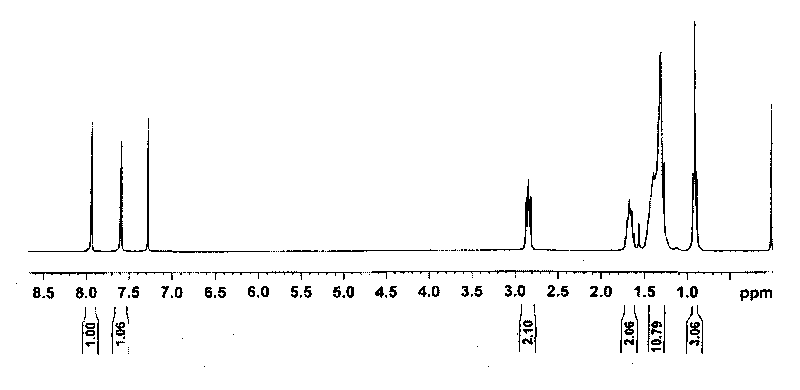
[0057] Dissolve dibenzothiophene (5g, 27.1mmol) and 0.1g iron powder in 30mL chloroform, avoid light, add 3.1mL liquid bromine at 0-5°C, and react at room temperature for 20h. Add saturated NaHSO 3 The excess bromine was removed by aqueous solution, and a pale yellow solid was obtained by filtration, washed twice with methanol, and recrystallized from chloroform to obtain a white solid. Yield: 80%. 1 H NMR (300MHz, CDCl 3 ) δ (ppm): 7.58 (m, 2H), 7.71 (m, 2H), 8.24 (m, 2H). Elemental analysis results. (%): C 12 h 6 S, calculated: C, 42.14; H, 1.77; S, 9.37; tested: C, 42.16; H, 1.83; S, 9.31.
[0058] 2)
[0059]
[0060] 2,8-dibromo-dibenzothiophene (6.48g, 20mmol) and 1g Ni(dppp)Cl 2 Dissolve in 200mL anhydrous tetrahydrofuran, avoid light, add the pre-prepared C 8 h 17 MgBr in diethyl ether (45 mmol). After the dropwise addition was completed, remove...
Embodiment 2
[0067] Example 2: Preparation of 2-octyl-3,7-dibromo-S, S-dioxy-dibenzothiophene
[0068] 1)
[0069]
[0070] Dissolve dibenzothiophene (10g, 54.2mmol) and 0.1g iron powder in 50mL of chloroform, avoid light, add liquid bromine (8.6g, 54.2mmol) at 0-5°C, and react at room temperature for 40h. Add saturated NaHSO 3 The excess bromine was removed in aqueous solution, filtered to give a pale yellow solid, and washed twice with methanol to give a white solid. Yield: 50%.
[0071] 2)
[0072]
[0073] 2-Bromo-dibenzothiophene (5.26g, 20mmol) and 1g Ni(dppp)Cl 2 Dissolve in 200mL anhydrous tetrahydrofuran, avoid light, add the prepared C dropwise below 10°C 8 h 17 MgBr ether solution (22 mmol). After the dropwise addition was completed, remove the ice bath, react at room temperature for 2 h, and use saturated NH 4 Cl to remove excess Grignard reagent, extracted with dichloromethane, and dried over magnesium sulfate. Carry out column chromatography with petroleum ethe...
Embodiment 3
[0080] Example 3: Preparation of 3,7-dioctyl-2,8-dibromo-S, S-dioxo-dibenzothiophene
[0081] 1)
[0082]
[0083] In a 150ml round bottom flask, biphenyl (5g, 32.5mmol) was dissolved in 80ml of methylene chloride, bromosuccinimide (NBS) (5.78g, 32.5mmol) was added at room temperature, and then reacted at room temperature 48 hours. After the reaction, the reactant was poured into water, extracted with dichloromethane, and washed with water several times. MgSO 4 Dry, distill off the solvent, and recrystallize from petroleum ether. A white solid was obtained, 5.65 g, yield: 75%.
[0084] 2)
[0085]
[0086]Add 4,4'-dibromobiphenyl (20g, 64.1mmol) to a 150mL three-necked flask, dissolve it in 50mL of chloroform, add chlorosulfonic acid (11.4mL, 171.7mmol) dropwise, and keep the reaction system at 50°C Below, the reaction is 3h. After the reaction was over, the reactant was poured into 500ml of crushed ice, and the ice was melted with NaCO 3 The solution was adjuste...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com