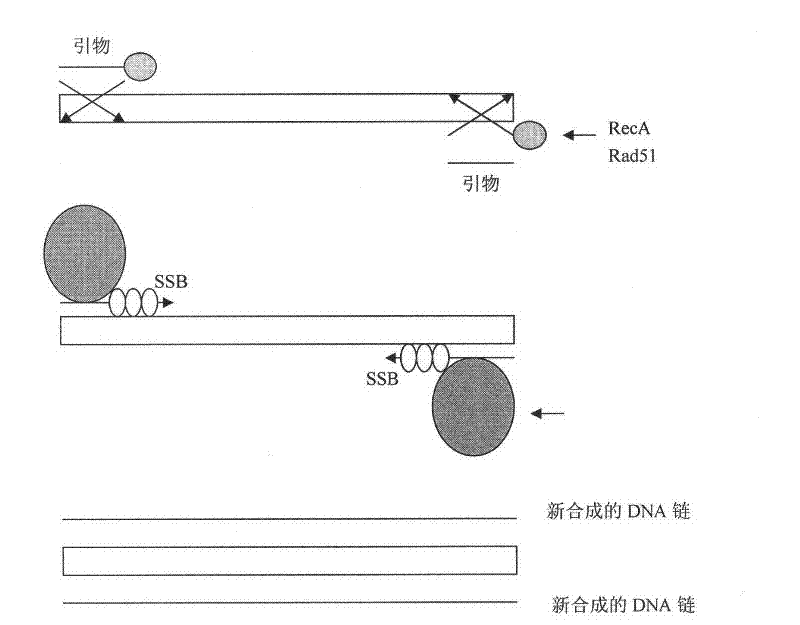
Reactive reagent of nucleic acid amplification by chain replacement at room temperature and nucleic acid amplification method at room temperature thereof
A technology of nucleic acid amplification and strand replacement reaction at room temperature, which is applied in the fields of nucleic acid amplification technology, molecular diagnosis and molecular biology, and can solve problems that limit the wide application of isothermal nucleic acid amplification technology, and achieve high-efficiency isothermal nucleic acid amplification and technical requirements Low, to avoid the effect of cross-contamination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Singleplex Nucleic Acid Amplification
[0039] This embodiment takes the blood detection and screening of HIV virus in blood as an example, and specifically describes the implementation process of traditional PCR and the single-plex normal temperature nucleic acid amplification of the present invention. The present invention can be but not limited to the detection of HIV viral nucleic acid molecules. Other viral diseases can also be tested in a similar way. For different pathogenic microorganisms, only the primer sequence needs to be redesigned. The primers in this example were all designed by the applicant and commissioned to be synthesized by Shanghai Sangong Company.
[0040] Specifically, it is achieved through the following steps: select the full sequence of HIV-1, and submit it to the database (http: / / www.ncbi.nlm.nih.gov / ) on the website of the National Center for Biological Sciences (http: / / www.ncbi.nlm.nih.gov / ) for Blast retrieval (click Click the Blast but...
Embodiment 2
[0084] multiplex nucleic acid amplification
[0085] This embodiment takes the blood detection and screening of HIV, HBV and HCV three common viruses in the blood as an example, and specifically describes the realization process of multiple strand replacement nucleic acid amplification. The present invention can detect, but is not limited to, these three viral nucleic acid molecules. Other viral diseases can also be detected in a similar way. In the embodiment, the RNA virus HIV and HCV are included, and the DNA virus HBV is also included. For different pathogenic microorganisms, only the primer sequence needs to be redesigned. The primers in this example were all designed by the applicant and commissioned to be synthesized by Shanghai Sangong Company.
[0086] Specifically, it is achieved through the following steps: select the full sequence of HIV-1, HBV and HCV genotype 1-6, and submit it segmentally to the database on the website of the National Center for Biotechnology...
Embodiment 3
[0184] This example takes HCV virus as an example to specifically illustrate the examples of the efficient nucleic acid amplification process achieved by strand replacement reaction temperatures of 25, 30, and 40° C. in the room temperature nucleic acid amplification method of the present invention. The primers in this example were designed by the applicant and commissioned to be synthesized by Shanghai Sangong Company.
[0185] The primers for the strand displacement reaction were designed as follows:
[0186] Forward primer 5'AATGGAACTATCTGATTCTACCGTGTCCCAAGTCA 3'SEQ ID NO: 14
[0187] Reverse primer 5' CTTAGATTTCCACGCCTTCAGTAAGAAGTCAACCC 3' SEQ ID NO: 15
[0188] In the biosafety cabinet, use 4M guanidine isothiocyanate solution to extract high-purity DNA and RNA from the patient's blood sample to obtain 50 μl of total nucleic acid sample containing DNA and RNA, which is labeled as sample 1; take 2 μl from sample 1 to a New small PCR tubes, using SuperScript VILO TM c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com