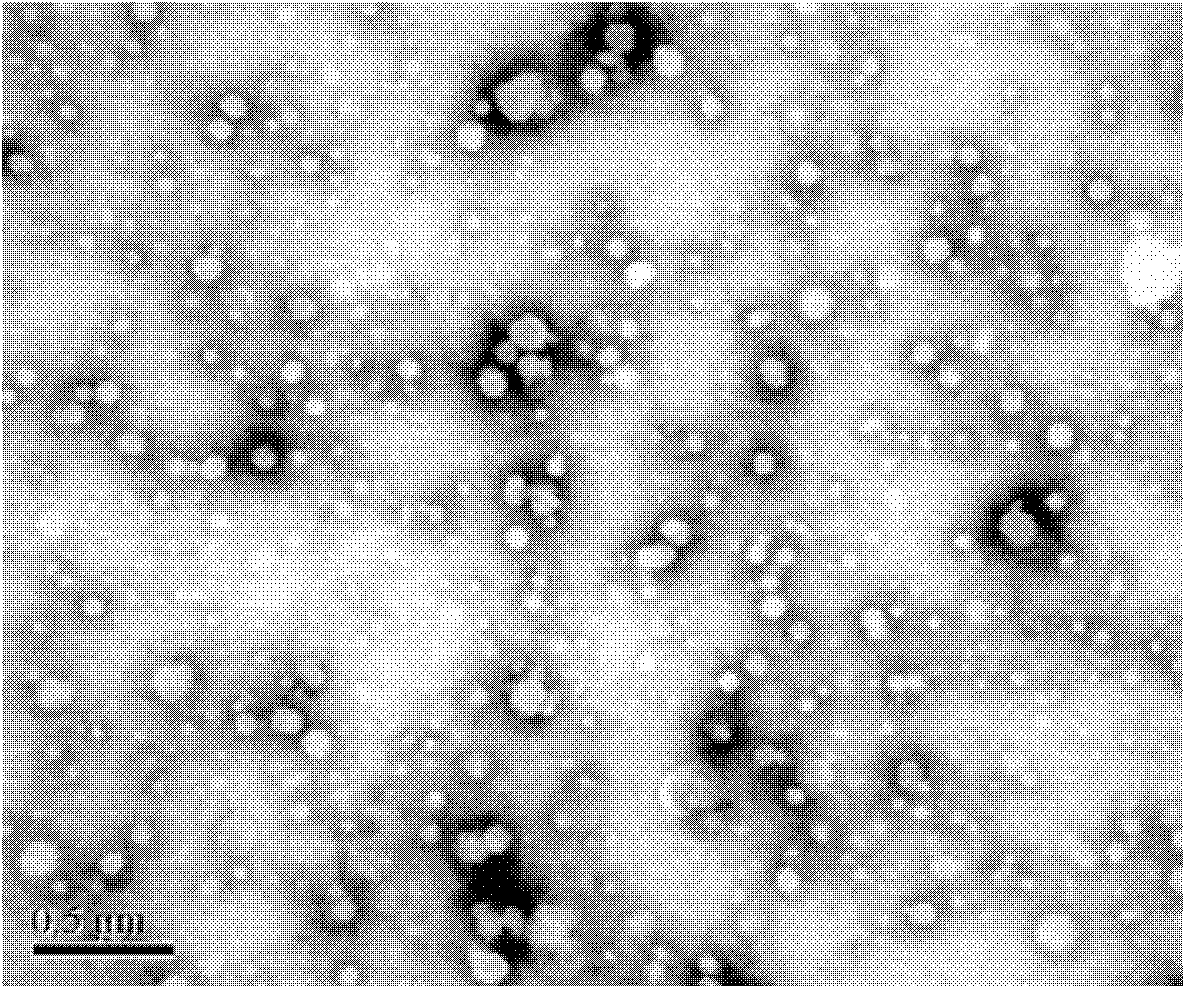
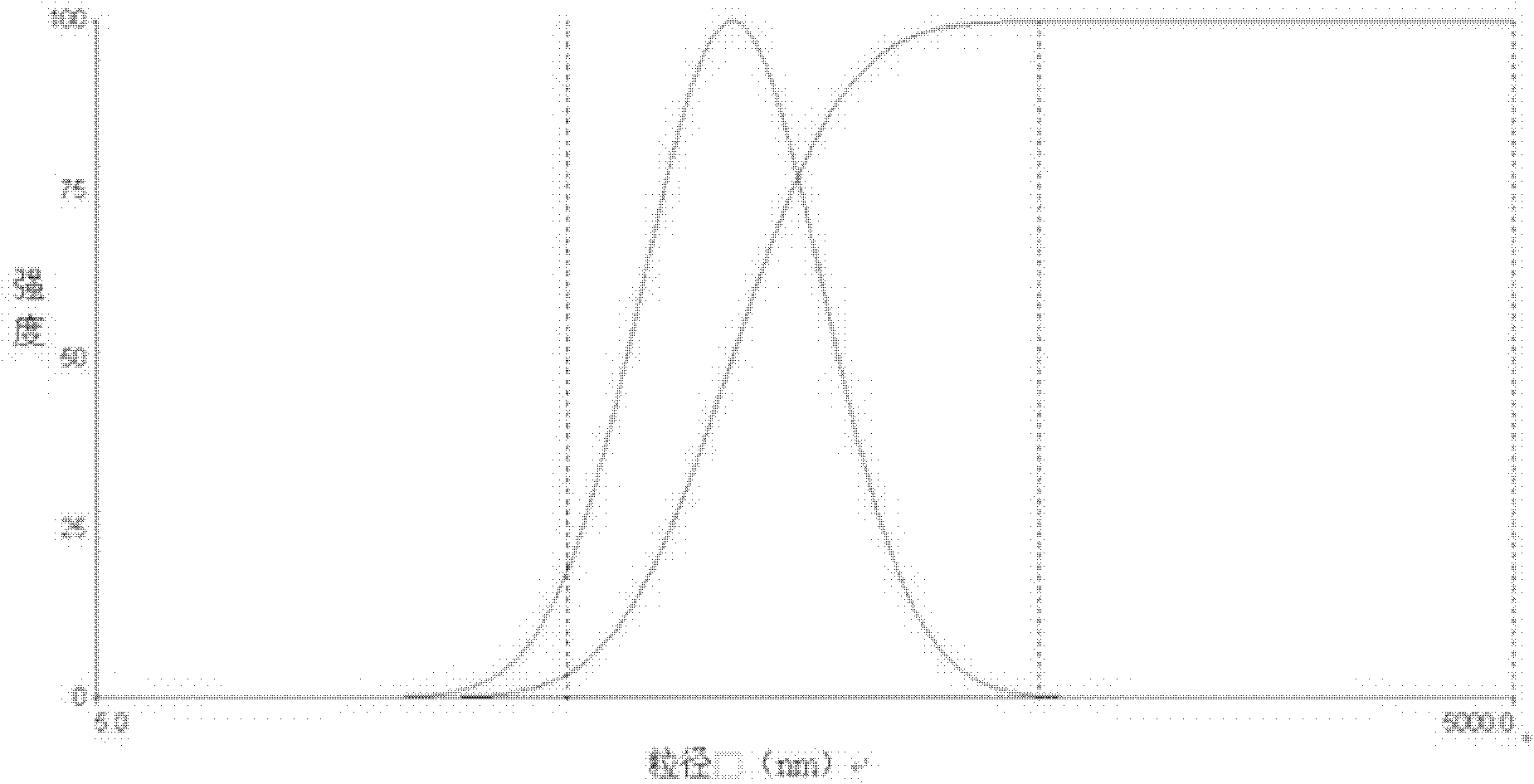
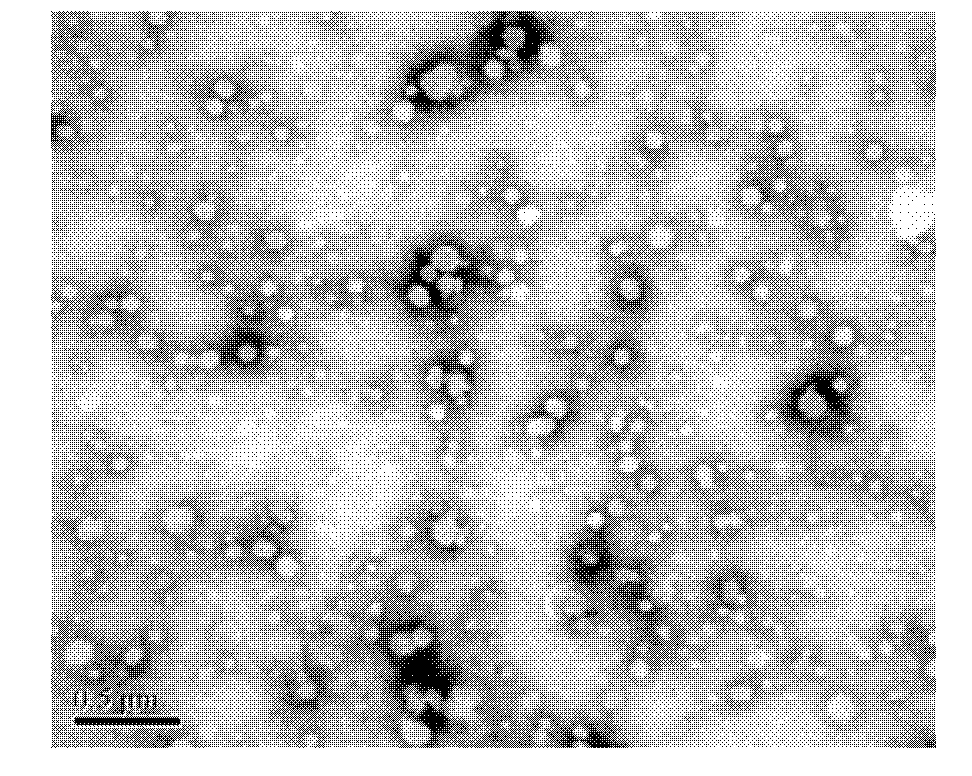
Mitoxantrone targeting sustained-release long-circulating nanometer liposome and preparation method
A long-circulation liposome and mitoxantrone technology, applied in the field of medicine, can solve the problems of easy fusion and aggregation during storage and application, poor physical and chemical stability, and low gene transfection efficiency, so as to increase bioavailability and biological Compatibility, adjustable release time, and short preparation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] d. Preparation of PEG-OQCMC: Dissolve the above-mentioned NHS-PEG in 100-150mL DMSO; dissolve the OQCMC prepared in the laboratory in 100-150mL water; -14,000 dialysis bags were dialyzed for one week, and finally filtered and freeze-dried to obtain the product.
[0032] In the preparation process of the FA-OQCMC, the contents of each component are: folic acid 3-10g; triethylamine 1.8-5ml; NHS 1.56-3g; NHS-FA 2.0-5.0g; OQCMC 15-30mg. The preparation process is as follows: dissolving folic acid in 60-100ml of DMSO solution, adding triethylamine to it, dissolving NHS with a certain amount of DMSO, adding it to the above reaction system and reacting in the dark for more than 12-20 hours. The product was filtered to obtain NHS-FA active lipid as a yellow solid. Dissolve NHS-FA active fat in 50-80ml DMSO, and add OQCMC. Adjust the pH of the reaction system to 10 with Na2HPO4 and NaOH buffer solution, and react for 1-1.5 h. The final product was dialyzed for 24 hours and fr...
Embodiment 1
[0034] The experimental process of PEG modified OQCMC:
[0035] a.Carboxylation of PEG: Dissolve 5.0g of PEG (2000Da) in 40mL of anhydrous toluene; then weigh 4.3g of maleic anhydride, add it to a four-necked flask under nitrogen protection, and heat it to 70°C for 48 , the process continued to stir. After the reaction, the organic solvent was distilled off under reduced pressure to obtain the preliminary product; diethyl ether was added for extraction, and then distilled under reduced pressure to obtain the product carboxylated PEG.
[0036]b.Activation of PEG: Weigh 0.75g of NHS and 0.3g of DCC for later use; weigh 2.8g of carboxylated PEG and NHS and dissolve in 40mL of chloroform, add DCC under the conditions of ice bath, magnetic stirring and nitrogen protection, React for 1 hour, then remove the ice bath, and react for 24 hours at room temperature; after the reaction, filter.
[0037] c. Purification of PEG: add the above filtered filtrate into 50 mL of acetone, place ...
Embodiment 2
[0042] The experimental process of PEG modified OQCMC:
[0043] a.Carboxylation of PEG: Dissolve 7.0g of PEG (2000Da) in 60mL of anhydrous toluene; weigh 7.2g of maleic anhydride, add it to a four-necked flask under nitrogen protection, and heat to 70°C for 50h , the process continued to stir. After the reaction, the organic solvent was distilled off under reduced pressure to obtain the preliminary product; diethyl ether was added for extraction, and then distilled under reduced pressure to obtain the product carboxylated PEG.
[0044] b. PEG activation: Weigh 0.8g of NHS and 0.4g of DCC for later use; weigh 3.5g of carboxylated PEG and NHS and dissolve them in 50mL of chloroform, add DCC under the conditions of ice bath, magnetic stirring and nitrogen protection, After reacting for 1.2h, remove the ice bath, and react at room temperature for 28h; after the reaction, filter.
[0045] c. Purification of PEG: add the filtered filtrate to 60 mL of acetone, place at 4°C for 2 ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com