Real-time fluorescence PCR ( Polymerase Chain Reaction) kit for detecting shigella and detection method thereof
A Shigella and kit technology, applied in the field of real-time fluorescent PCR kits, can solve the problems of low positive rate, long time-consuming, complicated operation, etc., and achieve the effects of good specificity, reduced false positive rate, and high detection sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Specific primers, fluorescent probes
[0044] 1. Materials:
[0045] Premix Ex Taq TM (2×) was purchased from TaKaRa Company (DRR039S);
[0046] MX3000P quantitative PCR instrument is a product of STRATAGNE, USA.
[0047] 2. Primer and probe design and synthesis:
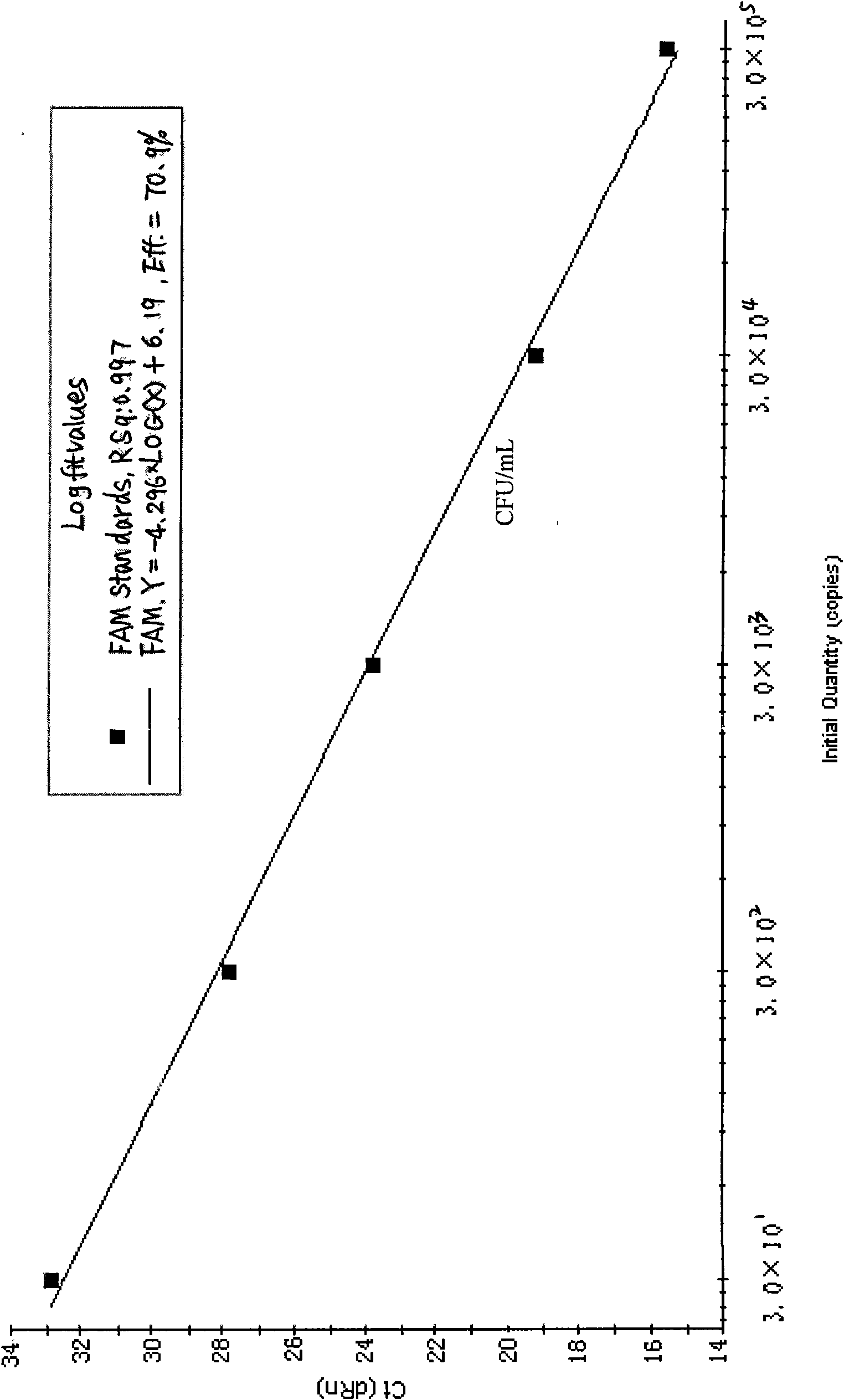
[0048] Using the Shigella ipaH gene sequence (registration number DQ448042) as a template, use PrimerExpressTM (V2.0, American ABI Company) software analyzes TaqMan-MGB primers and probe sites, and selects the best combination:
[0049] Upstream primer: 5′-GGAAAACCCTCCTGGTCCAT-3′
[0050] Downstream primer: 5′-CGCCGGTATCATTATCGAAAA-3′
[0051] Fluorescent probe: 5′-FAM-AGGCATCAGAAGGC-MGB-3′
[0052] Wherein FAM is a fluorescent reporter group, and MGB is a fluorescent quencher group.
[0053] Both were synthesized by Dalian Bao Biological Engineering Co., Ltd.
Embodiment 2
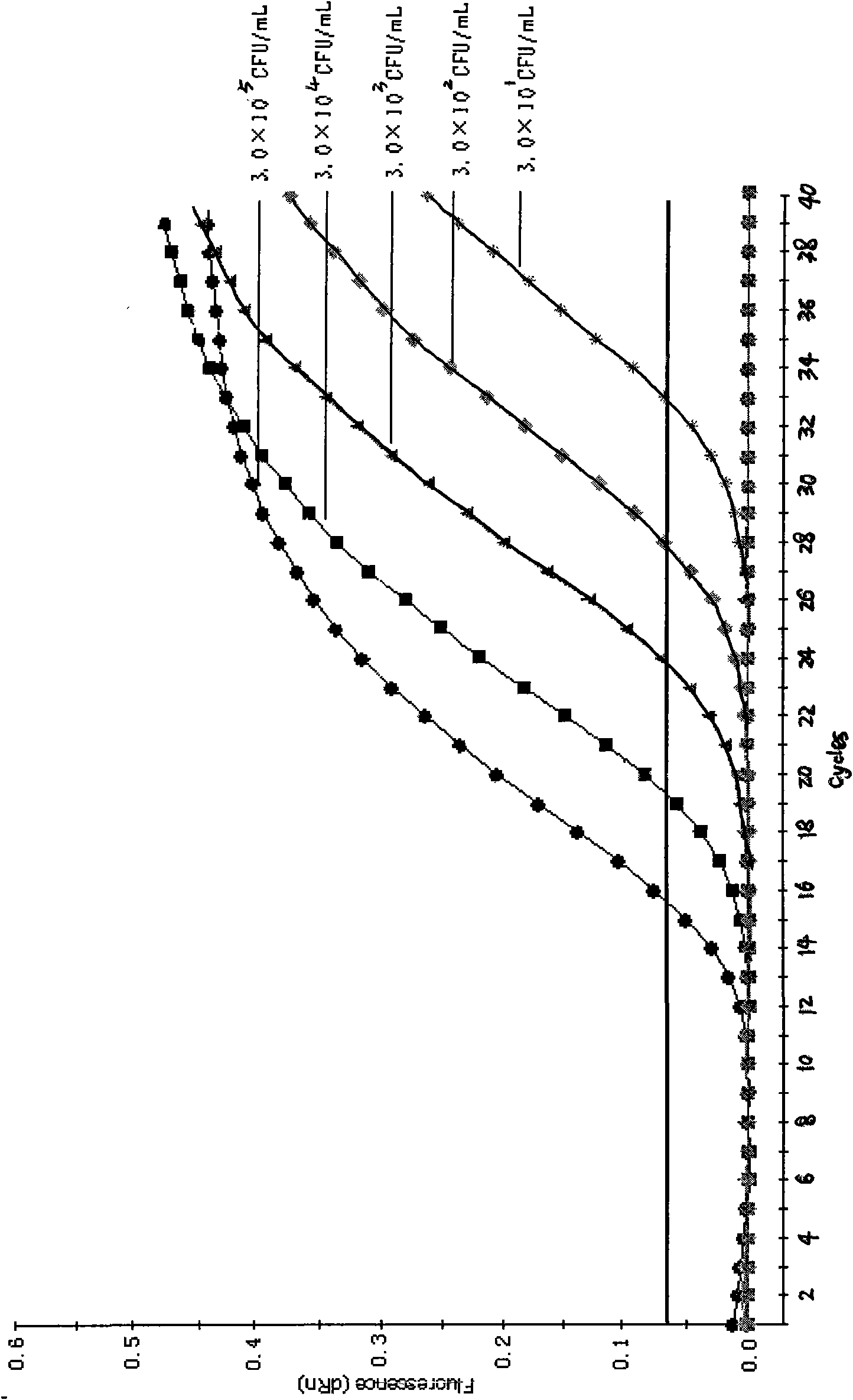
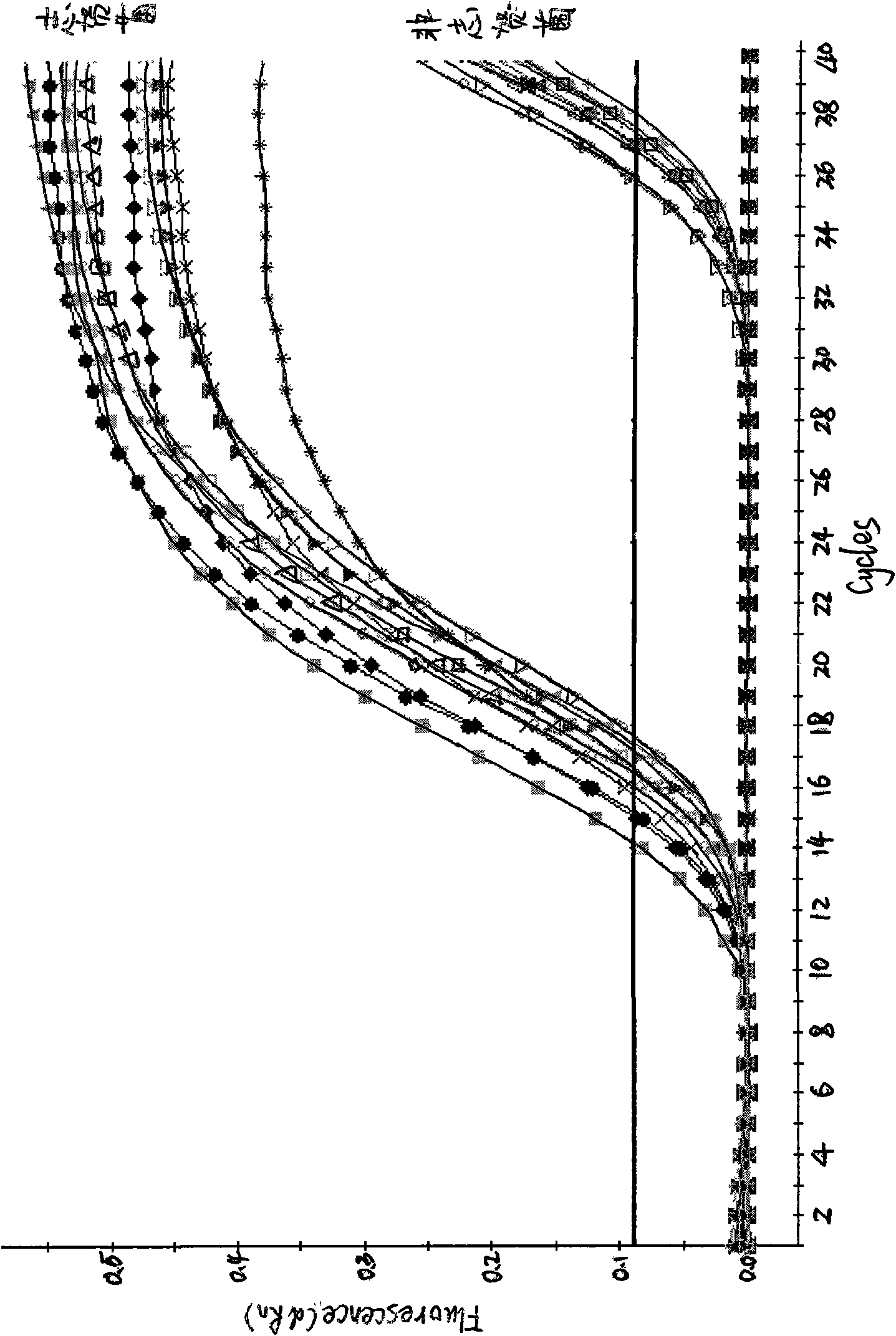
[0054] Embodiment 2: Specificity evaluation of Shigella fluorescent quantitative PCR method
[0055] 1. Strain detection:
[0056] Three Shigella standard strains (Shigella dysenteriae CMCC 51570, Shigella sonnei CMCC51334, Shigella flexneri ATCC 12022), 30 strains of Shigella isolates, Escherichia coli, Salmonella, Citrobacter, Escherichia coli, Vibrio parahaemolyticus, Yersinia, Proteus, Staphylococcus epidermidis, Staphylococcus aureus, Streptococcus pyogenes, Alcaligenes faecalis, Lactobacillus lymannii, Bacillus cereus, Salmonella anativa, Streptococcus mirabilis Proteus, hemolytic streptococcus and other 91 strains of non-Shigella bacteria suspension were extracted by pyrolysis method, and then PCR amplification was carried out on the MX3000P quantitative PCR instrument of American STRATAGNE company with the upstream and downstream primers and probes for detection.
[0057] (1) Thermal cracking method: collect the strains in a 1.5mL tube, place in a boiling water bath f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com