Synthesis method of 2-nitro-4-substituted phenylacetic acid
A synthesis method, 2-X-5- technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems that are not suitable for industrial production, high production equipment requirements, difficult to control the amount of added equivalents, etc. problem, to achieve the effect of saving preparation cost, no synthesis conditions, and improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
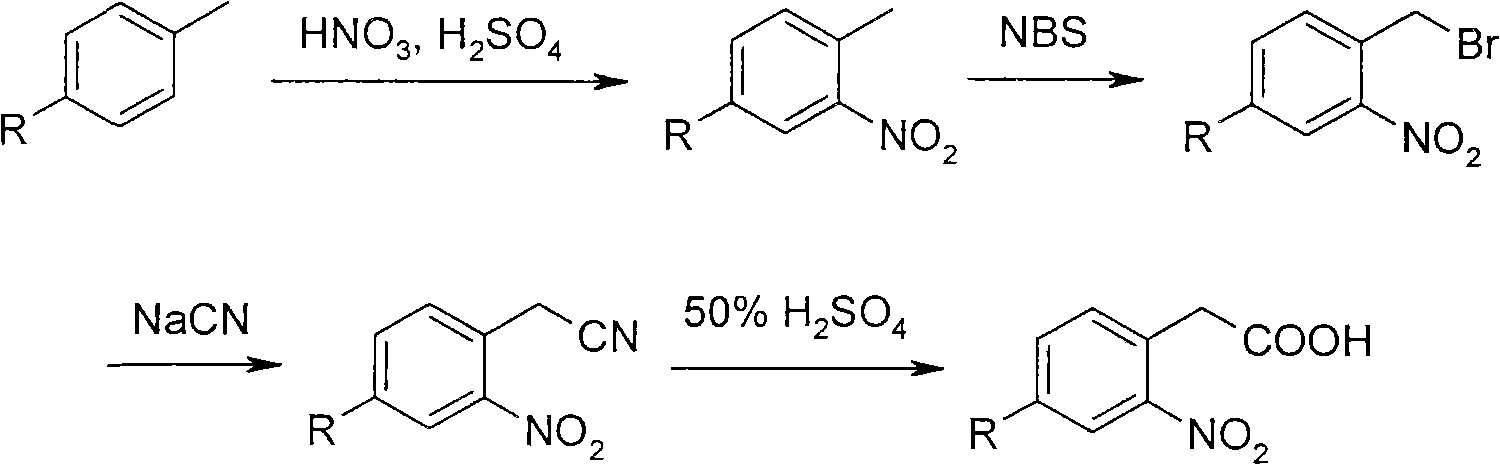
[0033] Synthesis of 2,5-dibromonitrobenzene (2).
[0034] In a 100ml three-necked flask equipped with a mechanical stirrer, a thermometer and a constant pressure dropping funnel, add 23.6g of p-dibromobenzene (1) (100mmol) and 50ml of methylene chloride, and slowly add 7.3g of 95% concentrated nitric acid (116mmol) and the mixed acid that 16ml98% concentrated sulfuric acid forms, keep temperature below 30 ℃, be warming up to 38 ℃ of reaction 1 hour after completion of dropwise addition, thin-layer chromatography (TLC) detects reaction terminal point.
[0035] Pour into 250ml of ice water after the reaction, the temperature will rise violently during the pouring process, control the temperature not to exceed 10°C, extract with dichloromethane to obtain the organic layer, evaporate the solvent, and recrystallize the solid with ethanol to obtain a yellow solid (2) 21.6 g, yield 90%, melting point 82 ℃ ~ 84 ℃, IR (KBr, cm -1 ) 1350, 1520 (-NO 2 ). Reaction formula:
[0036] ...
Embodiment 2
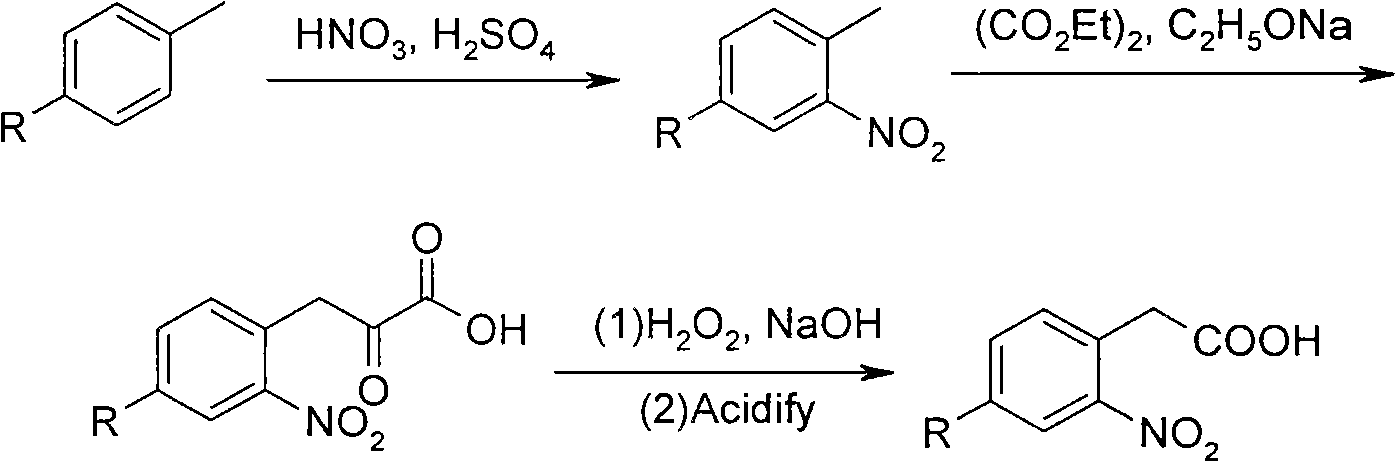
[0047] Synthesis of 2,5-difluoronitrobenzene (2').
[0048] In a 100ml three-necked flask equipped with a mechanical stirrer, a thermometer and a constant pressure dropping funnel, add 11.4g of p-difluorobenzene (1') (100mmol) and 50ml of dichloromethane, slowly dropwise add 7.3g of 95% concentrated The mixed acid of nitric acid (116mmol) and 16ml 98% concentrated sulfuric acid is formed, keep temperature below 30 ℃, be warming up to 38 ℃ and react for 1 hour after completion of dropwise addition, thin-layer chromatography (TLC) detects the end point of reaction.
[0049] After the reaction, pour into 250ml of ice water. During the pouring process, the temperature will rise violently. Control the temperature not to exceed 10°C. Extract the organic layer with dichloromethane. After evaporating the solvent, distill under reduced pressure to obtain a colorless transparent liquid (2') 13.2g, yield 83%, boiling point 3mmHg / 60 ℃, IR (KBr, cm -1 ) 1350, 1520 (-NO 2 ). Reaction for...
Embodiment 4
[0071] Embodiment 4: industrial scale-up test according to example 1.
[0072] 2, the synthesis of 5-dibromonitrobenzene (2), under the same operating process, 50 kg of p-dibromobenzene (1) was charged to obtain 47 kg of 2,5-dibromonitrobenzene (2).
[0073] The synthesis of 2-nitro-4-bromophenylacetonitrile (4), under the same operation process, 2,5-dibromonitrobenzene (2) feeds 47 kilograms, can obtain 2-nitro-4- Bromophenylacetonitrile and 15 kg of 2-nitro-4-bromophenylacetic acid.
[0074] The synthesis of 2-nitro-4-bromophenylacetic acid (5), under the same operation process, feeds 21 kilograms of 2-nitro-4-bromophenylacetonitrile, can obtain 22 kilograms of 2-nitro-4-bromobenzene acetic acid. Chemical analysis and test results are basically the same as Example 1.
[0075] For those skilled in the art, under the inspiration of the patent concept and specific embodiments, some deformations that can be directly derived or associated from the patent disclosure and common ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com