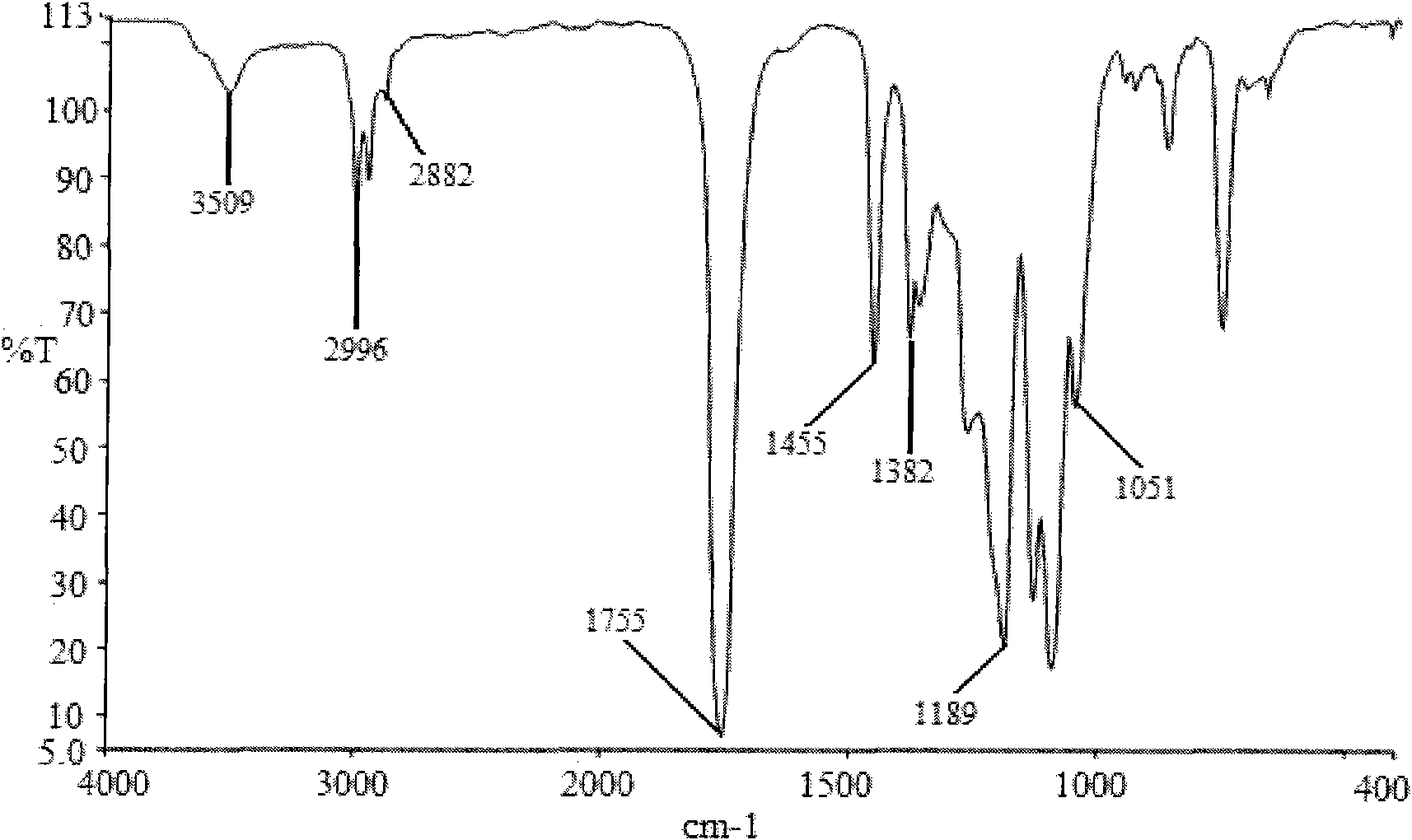
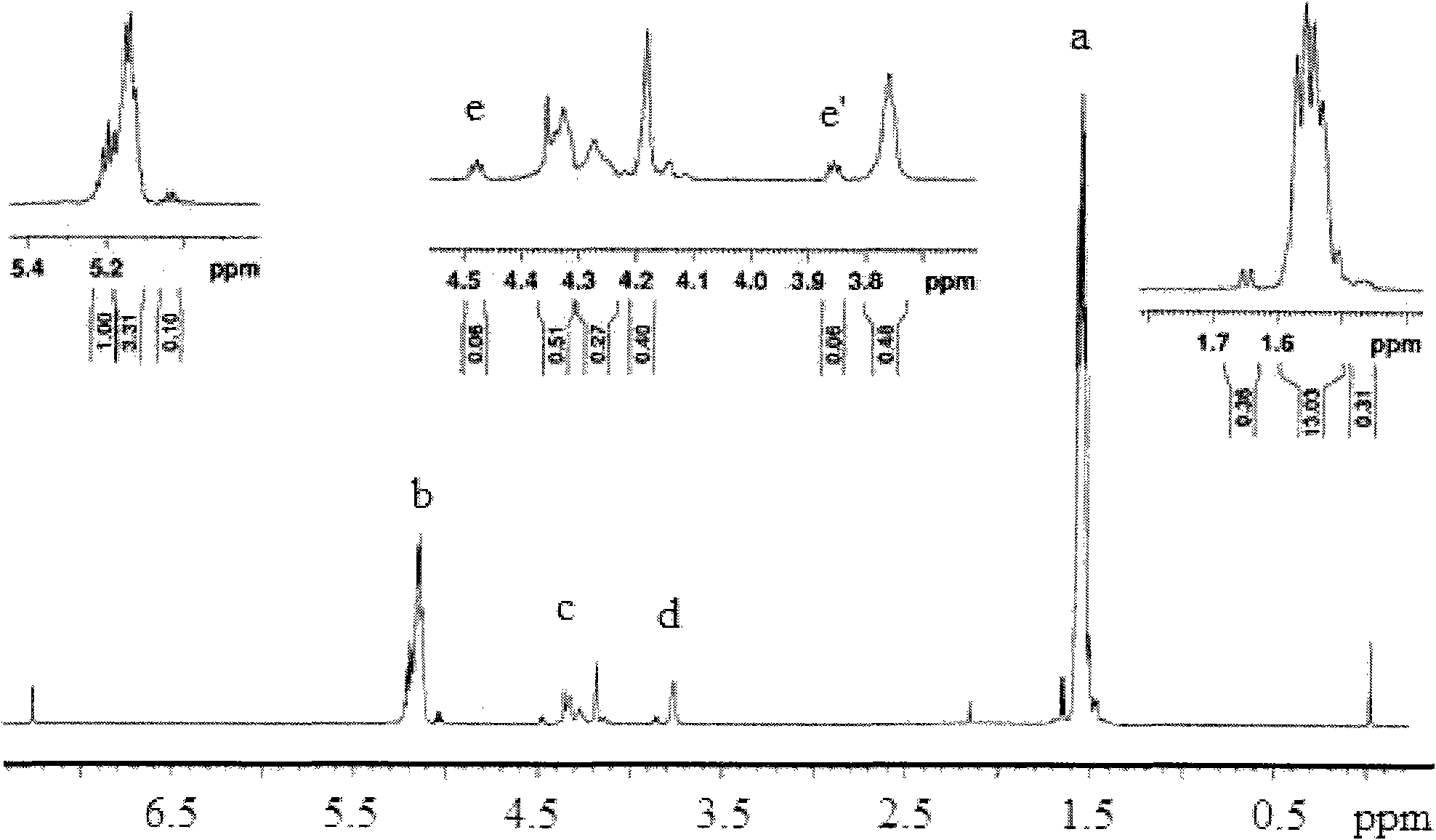
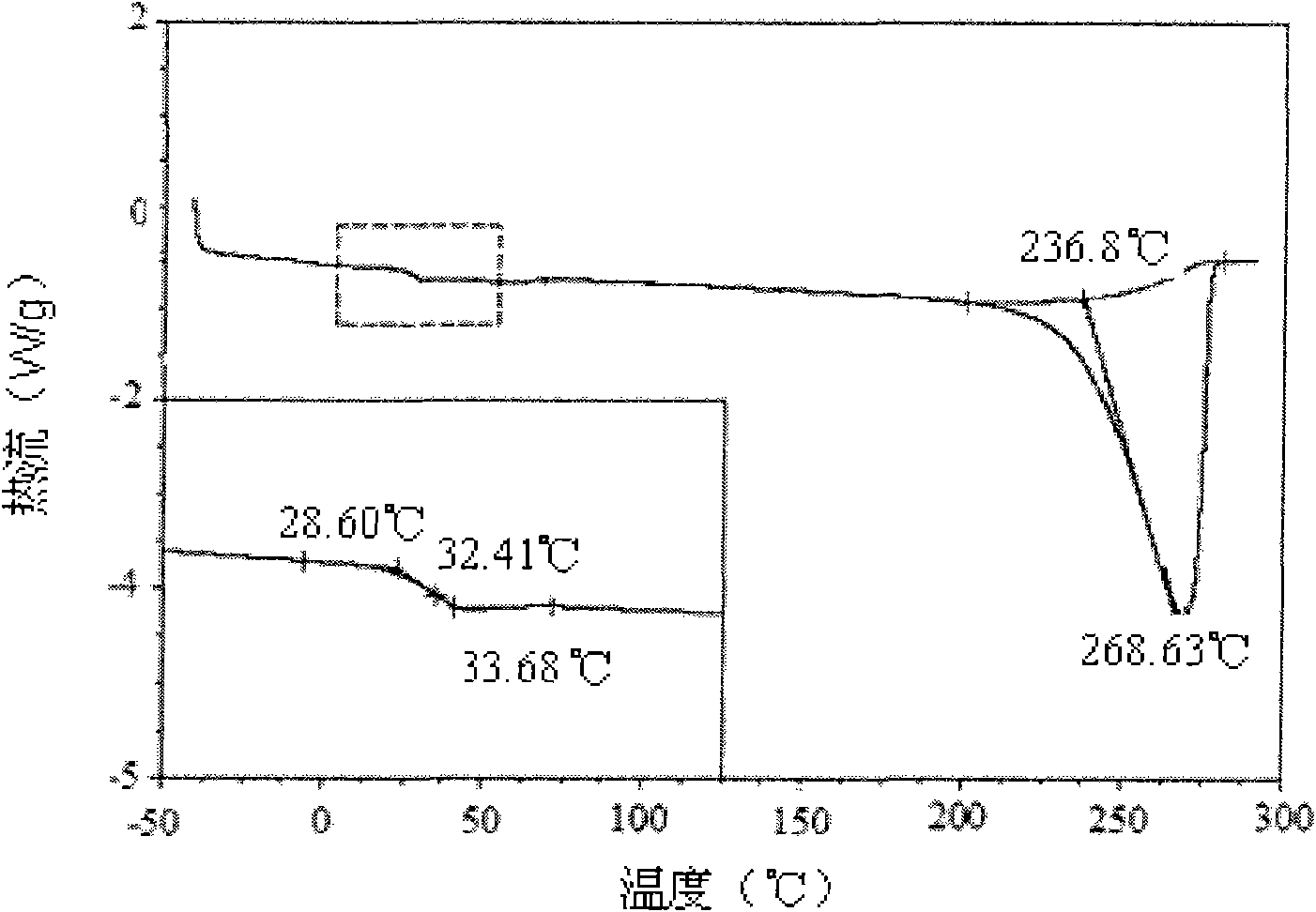
Preparation method of shape memory polyurethane based on lactide and 1, 4-p-dioxanone
A technology for dioxanone and lactide, which is applied in the field of preparation of shape memory polymers, can solve the problems of difficulty in obtaining high molecular weight chain shape memory polyurethane, low yield of final products, poor reaction controllability and the like , to prevent the initiation of cross-linking reaction, improve the controllability of the reaction, and improve the product yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] a, the preparation of hydroxyl-terminated poly(lactide-co-1,4-dioxanone)
[0031] First, 16 μL (5.36×10 -6 mol) and ethylene glycol 32μL (5.73×10 -4mol) mixed evenly, evacuated for 10-20 minutes, sealed, and reacted at 80°C for 24 hours (use gas chromatography to detect the remaining amount of monomer to monitor the reaction process), cool, break the vacuum, and then add D, L-lactide 5.000 g (0.03472mol) (the molar ratio of D,L-lactide to 1,4-dioxanone is 0.7:1) and stannous octoate T-9 at a concentration of 0.1347g / mL in dichloromethane Solution 16μL (5.36×10 -6 mol), mix evenly, vacuumize for 10-20 minutes, seal, react at 140°C for 24 hours, cool, break the vacuum, dissolve the reaction product in dichloromethane, purify with absolute ethanol, and dry in vacuum at room temperature to obtain hydroxyl-terminated poly( Lactide-co-1,4-dioxanone) 3.8g, the average molecular weight recorded by the terminal hydroxyl analysis method is 5000, and the T is recorded by the DS...
Embodiment 2
[0041] The main difference between this example and Example 1 is that in step a, by adjusting the molar ratio of D, L-lactide to 1,4-dioxanone, the hydroxyl-terminated poly(lactide) Ester-co-1,4-dioxanone) average molecular weight and glass transition temperature.
[0042] a, the preparation of hydroxyl-terminated poly(lactide-co-1,4-dioxanone)
[0043] First, 1.25 mL (0.01551 mol) of 1,4-dioxanone and 16 μL (5.36×10 -6 mol) and ethylene glycol 32μL (5.73×10 -4 mol) mixed evenly, evacuated for 10-20 minutes, sealed, reacted at 80°C for 12 hours, cooled, broke the vacuum, and then added D, L-lactide 5.000g (0.03472mol) (D, L-lactide and 1 , the molar ratio of 4-dioxanone is 2.24:1) and 16 μL (5.36×10 -6 mol), mix evenly, vacuumize for 10-20 minutes, seal, react at 130°C for 24 hours, cool, break the vacuum, dissolve the reaction product in dichloromethane, purify with absolute ethanol, and dry in vacuum at room temperature to obtain hydroxyl-terminated poly( Lactide-co-1,4-...
Embodiment 3
[0047] The main difference between this example and Example 2 is that in step b, by adjusting hydroxyl-terminated poly(lactide-co-1,4-dioxanone), 1,6-hexamethylene The molar ratio of diisocyanate to stannous octoate and the molar ratio of butanediamine to hydroxyl-terminated poly(lactide-co-1,4-dioxanone), thereby adjusting the hydroxyl-terminated poly(lactide-co - the glass transition temperature of 1,4-dioxanone)-based block polyurethane.
[0048] a, the preparation of hydroxyl-terminated poly(lactide-co-1,4-dioxanone) (same as Example 2)
[0049] b, preparation of hydroxyl-terminated poly(lactide-co-1,4-dioxanone)-based block polyurethane
[0050] First, 5 g (0.001 mol) of hydroxyl-terminated poly(lactide-co-1,4-dioxanone) obtained in step a, 184 μL (0.0015 mol) of 1,6-hexamethylene diisocyanate and a concentration of 30μL of 0.1347g / mL stannous octoate T-9 in dichloromethane -5 mol) (the molar ratio of the three is 1:1.5:0.01) dissolved in 15mL of toluene that has been ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Tg | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com