Method for preparing manganese phosphate material
A technology of manganese phosphate and phosphate, which is applied in the field of inorganic preparation of phosphate functional materials, can solve the problems of complex process, small product quantity per batch, high energy consumption under high temperature and high pressure, and achieve simple process, less production process, single The effect of high secondary yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Mn(H 2 PO 4 ) 2 Solution reflux reaction to prepare phosphomanite [Mn(HPO 4 ) 2 (PO 4 ) 2 (H 2 O)]
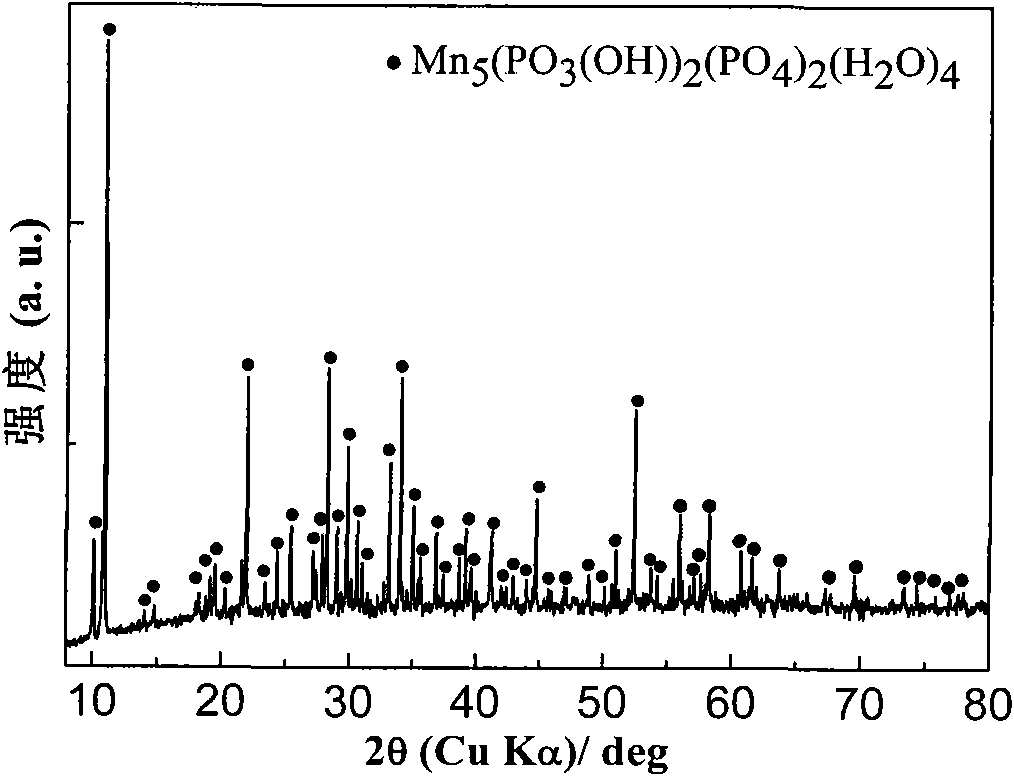
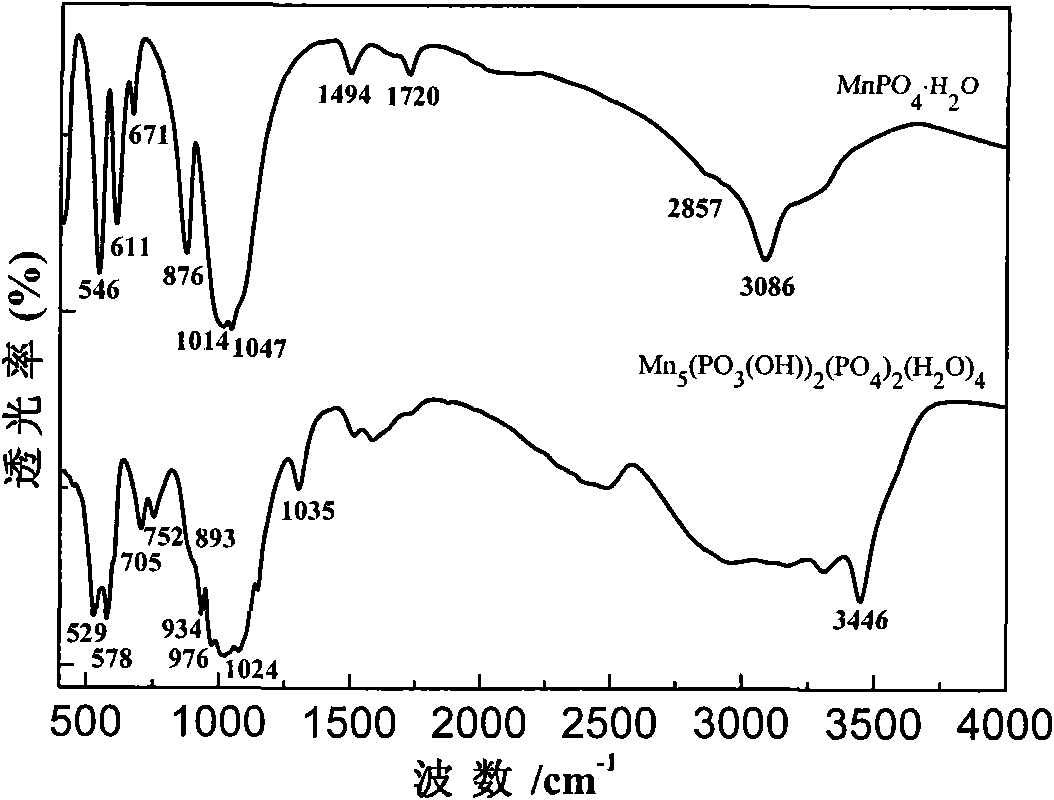
[0041] Weigh 30mmol Mn(H 2 PO 4 ) 2 2H 2 O in the Erlenmeyer flask, add 100ml distilled water and be mixed with the Mn(H of 0.3mol / L 2 PO 4 ) 2 2H 2 O solution, placed in a heat-collecting constant temperature heating magnetic stirrer (its stirring speed is 30 rpm) refluxed at 100 ° C for 12 hours, took out and filtered after natural cooling, and the reaction product was washed with de-distilled water or deionized water until the conductivity of the filtrate was Just less than 30μS / cm. The product was dried in an oven at 60°C, and its crystal structure was characterized by X-ray diffractometer (XRD) (see figure 1 ), Fourier transform infrared spectroscopy (FTIR) to analyze its main functional groups (see figure 2 ) and scanning electron microscopy to characterize its morphology (see image 3 ), the result showed that the obtained product ...
Embodiment 2
[0043] Embodiment 2: with Mn (H 2 PO 4 ) 2Synthesis of manganese phosphate monohydrate (MnPO 4 ·H 2 O)
[0044] Weigh 30mmol Mn(H 2 PO 4 ) 2 2H 2 O in the Erlenmeyer flask, add 100ml distilled water and be mixed with the Mn(H of 0.3mol / L 2 PO 4 ) 2 2H 2 O solution, further add 80 and 120mmol concentrated hydrochloric acid (concentration ≥ 36%) respectively, then add 60mmol NaClO solution, place the collector type constant temperature heating magnetic stirrer (its stirring speed is 30 rpm) at 100 ℃ Reflux for 12 hours, take out and cool naturally, then filter, and wash the reaction product with deionized water until the conductivity of the filtrate is less than 30 μS / cm. The product was dried in an oven at 60°C, and its crystal structure was characterized by XRD (see Figure 7 ), FTIR analysis of its main functional groups (see figure 2 ) and scanning electron microscopy to characterize its morphology, the results showed that the product was manganese phosphate m...
Embodiment 3
[0045] Embodiment 3: with MnCl 2 Synthesis of manganite and manganese phosphate monohydrate as sources of divalent manganese
[0046] Weigh 30mmol MnCl 2 4H 2 O and 60mmol NaClO solution in a conical flask, add 100mL distilled water to prepare a solution, add 30, 60, 90, 135 and 180mmol H 3 PO 4 Solution, placed in a heat-collecting constant temperature heating magnetic stirrer (its stirring speed is 30 rpm) at 60, 80 and 100 ° C for reflux reaction for 12 hours, took out and filtered after natural cooling, and the reaction product was washed with deionized water until the filtrate conductivity Just less than 30μS / cm. The product was dried in an oven at 60°C, its crystal structure was characterized by XRD, and its main functional groups were analyzed by FTIR. The results showed that adding 30 mmol H 3 PO 4 solution, the products obtained at the three reaction temperatures are all pyrosite, and adding H 3 PO 4 The solution amounts are 60, 90, 135 and 180 mmol, and the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com