Method for producing 3,6-dichloropyrimidine-2-carboxylic acid by biocatalysis
A dichloropyridine, biocatalysis technology, applied in microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve problems such as serious environmental pollution, and achieve high yield, mild reaction conditions, and environmentally friendly effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 strain screening
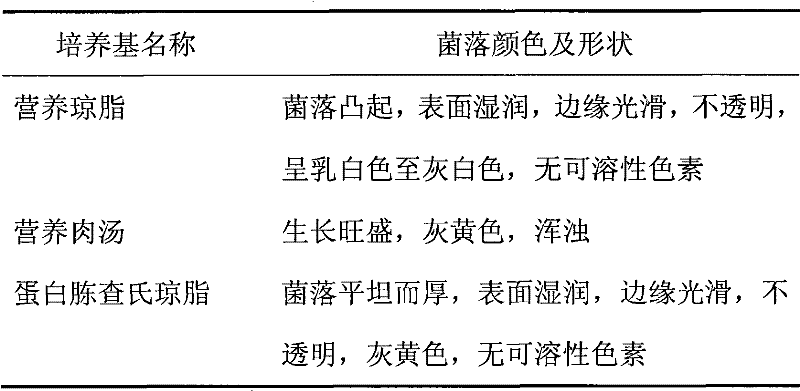
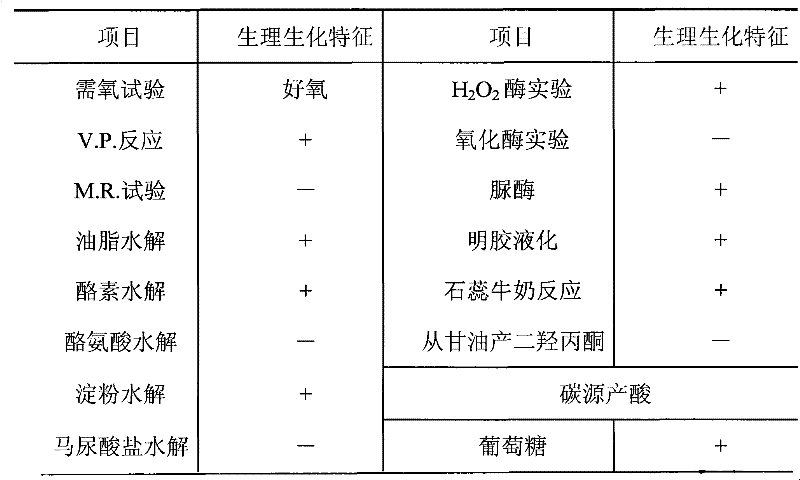
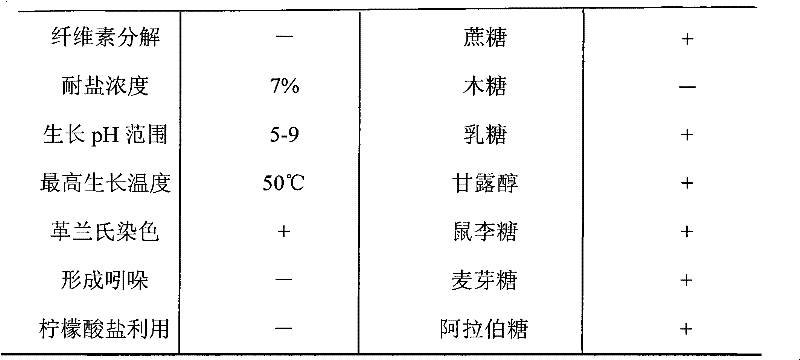
[0036]The Bacillus subtilis (Bacillus subtilis) KR2 involved in the present invention is screened by members of the biochemical research group of our company from the soil of pesticide factories polluted by nitrile compounds for more than 15 years.
[0037] The screening method of bacterial classification among the present invention is as follows:
[0038] More than 100 batches of sludge samples were taken from different angles of the sewage pool of Nanjing No. 1 Pesticide Factory, and screened and identified after enrichment and cultivation in batches:
[0039] Weigh 10 grams of sludge, add 50 mL of sterile water, shake it with glass beads, inoculate 5 mL of the suspension into 45 mL of enrichment medium, place on a shaker at 30°C, and shake at 120 rpm for 3 days. Afterwards, the culture solution was taken for gradient dilution, and 10 -6 、10 -7 、10 -8 Gradient dilutions were spread on the plate containing the first screening solid m...
Embodiment 2
[0045] The preparation of embodiment 2 biocatalysts
[0046] Scrape 3 loops of Bacillus subtilis KR2 (CGMCC No.3242) slant strains with an inoculation loop in a sterile ultra-clean workbench, and inoculate them into the primary liquid seed medium (glucose: 15, yeast extract: 5, peptone: 5. Sodium chloride: 2, potassium dihydrogen phosphate: 1, dipotassium hydrogen phosphate: 1, magnesium sulfate: 0.5, caprolactam: 5, isovaleronitrile: 1, cobalt chloride: 10ppm, pH: 7.0, unit: g / L). At a temperature of 25°C and a rotating speed of 150rpm, the seed liquid of the first-grade Bacillus subtilis KR2 (CGMCC No.3242) can be obtained by shaking and culturing for 48 hours, and the first-grade seed liquid is transferred to the same The culture is continued in the sterilized medium, and the secondary seed liquid of Bacillus subtilis KR2 (CGMCC No. 3242) can be obtained after 48 hours.
[0047] Inoculate the secondary seed liquid of Bacillus subtilis KR2 (CGMCC No.3242) into 35L sterili...
Embodiment 3
[0049] Example 3 Preparation of 3,6-dichloro-2-cyanopyridine
[0050] In a 50L stirred tank, a two-phase system consisting of water-ethyl acetate was used as the medium for the entire catalytic reaction. 3Kg of 3,6-dichloro-2-cyanopyridine was dissolved in 15L of ethyl acetate as the upper organic phase of the catalytic reaction. Add 1Kg of Bacillus subtilis KR2 (CGMCC NO.3242) wet cells to 20L of deionized water as a biocatalyst as the lower aqueous phase of the reaction system. Firstly, 5 L of the ethyl acetate organic phase containing the substrate was mixed with 20 L of the aqueous phase containing the biocatalyst, the temperature of the reaction system was maintained at 30° C., and the reaction was started by stirring at 150 rpm. Afterwards, 5 L of the ethyl acetate solution in which 3,6-dichloro-2-cyanopyridine was dissolved was added every 8 hours for a total of 2 times, and the reaction was terminated after 30 hours of reaction.
[0051] After releasing the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com