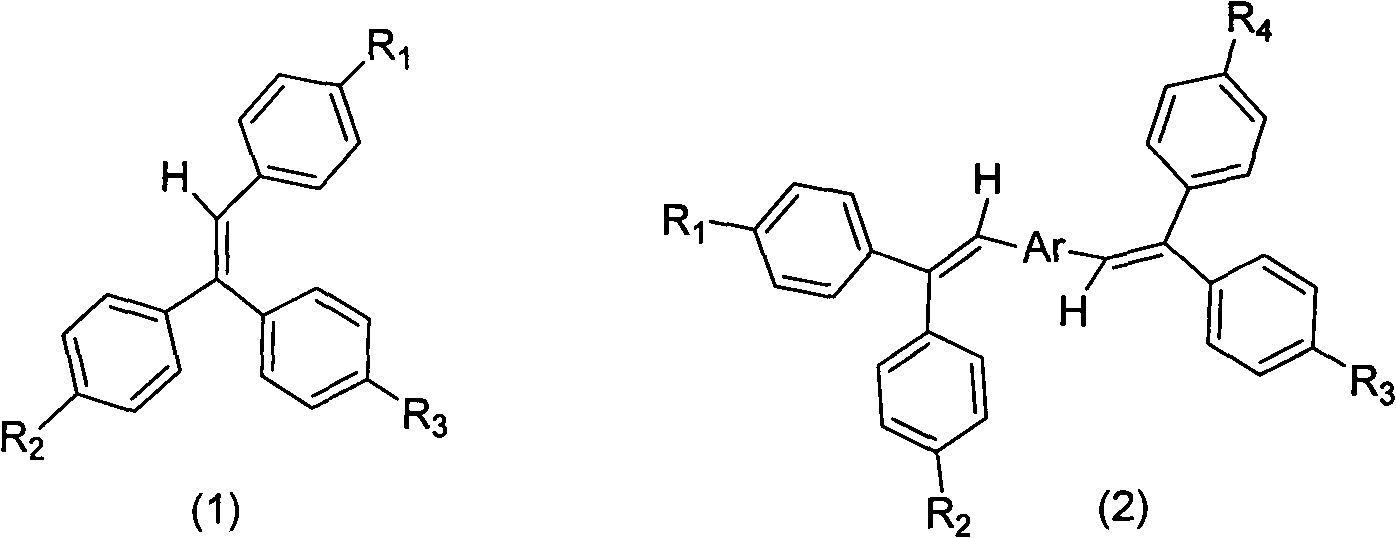
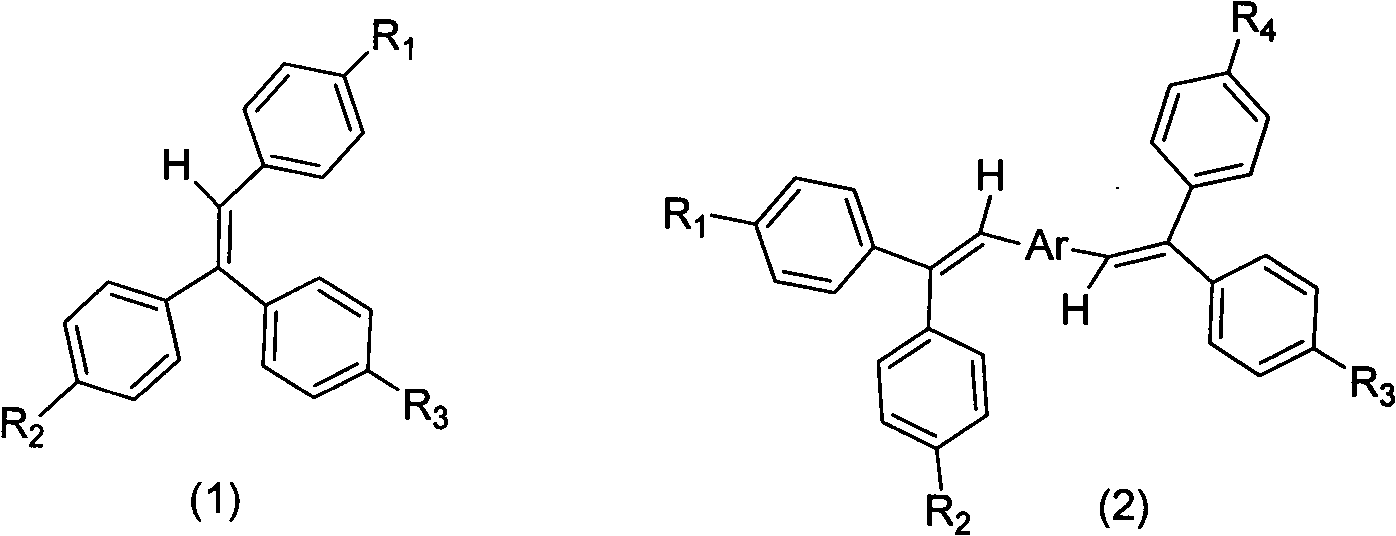
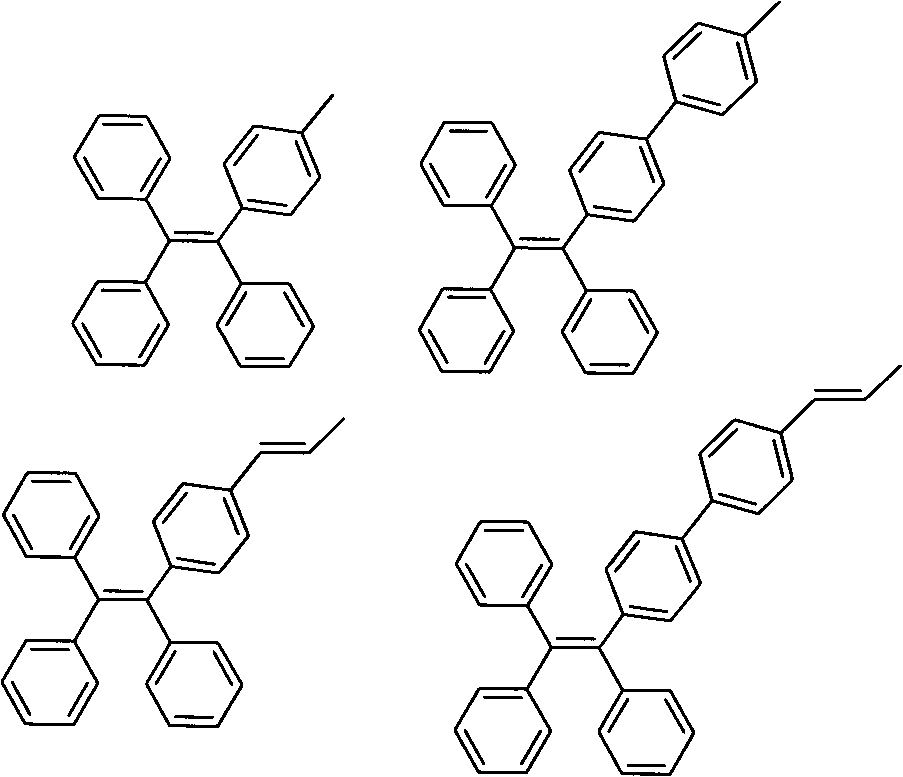
Aggregation-induced light emitting material simultaneously containing triphenylethylene structure and tetraphenylethylene structure and synthesis method and application thereof
A technology of aggregation-induced luminescence and tetraphenylethylene, applied in the direction of luminescent materials, chemical instruments and methods, hydrocarbons, etc., can solve the problems of aggregation fluorescence quenching, etc., and achieve the effect of easy purification, high thermal stability, and high luminous intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The synthesis method of above-mentioned luminescent material, comprises the following steps:
[0022] Step 1: Synthesis of triphenylethylene intermediates with functional groups
[0023] The synthesis of triphenylethylene intermediates with functional groups adopts the reaction of benzophenone and its halogenated products with halogenated phenylphosphonate ylide reagents to form halogenated triphenylethylene intermediates. Preferred benzophenone raw material of the present invention is 4,4'-dibromobenzophenone, 4-bromobenzophenone and benzophenone; Preferred ylide reagent is 4-bromobenzylidene phosphonic acid diethyl ester. The preparation process for synthesizing the triphenylethylene intermediate from the above preferred raw materials is simple and the yield is high, which is one of the main features of the present invention. For the synthesis of substituents, conventional organic synthesis methods are used, including Friedel-Crafts alkylation, amine alkylation, halo...
Embodiment 1
[0036] Triphenylethylene-tetraphenylethylene [VP 3 -(TPE) 1 ]Synthesis:
[0037] (1) Bromotriphenylethylene (VP 3 -Br) synthesis
[0038] Dissolve benzophenone (13.59g, 74.6mmol) and diethyl 4-bromobenzylidene phosphonate (27.49g, 89.5mmol) in 250mL THF, add potassium tert-butoxide (10.01g, 89.5mmol) , Stir the reaction at room temperature under the protection of argon for 4h. The solvent was evaporated under reduced pressure, the sample was purified by silica gel column chromatography, and the eluent was n-hexane to obtain 23.20 g of white crystals with a yield of 93%.
[0039]
[0040] (2) Synthesis of brominated tetraphenylethylene (TPE-Br):
[0041]Dissolve diphenylmethane (4.00g, 23.8mmol) in 50mL tetrahydrofuran, stir for 30min at 0°C under argon protection, slowly drop into 2.2M n-hexane solution of n-butyllithium (13.0mL, 28.6mmol), stir Reaction 1h. Then 4-bromobenzophenone (4.97g, 19.0mmol) was added, the temperature was slowly raised to room temperature, a...
Embodiment 2
[0050] Triphenylethylene-bis(tetraphenylethylene)[VP 3 -(TPE) 2 ]Synthesis:
[0051] (1) Dibromotriphenylethylene (VP 3 -Br 2 )Synthesis:
[0052] Dissolve 4,4'-dibromobenzophenone (2.00g, 5.8mmol) and diethyl benzylidene phosphonate (1.98g, 8.7mmol) in 40mL tetrahydrofuran, add potassium tert-butoxide (0.98g , 8.7mmol), and stirred at room temperature for 4h under the protection of argon. The solvent was evaporated under reduced pressure, and the sample was purified by column chromatography using n-hexane as the mobile phase and silica gel as the stationary phase to obtain 2.10 g of white crystals with a yield of 88%.
[0053]
[0054] (2) Triphenylethylene - two (tetraphenylethylene) [VP 3 -(TPE) 2 ]Synthesis:
[0055] put VP 3 -Br 2 (0.22g, 0.53mmol) and TPEB (0.48g, 1.3mmol) were dissolved in 30mL of toluene, 2M potassium carbonate aqueous solution (0.7mL) and 5 drops of Aliquat336 were added, and after argon flow for 40min, a catalytic amount of Pd(PPh 3 ) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com