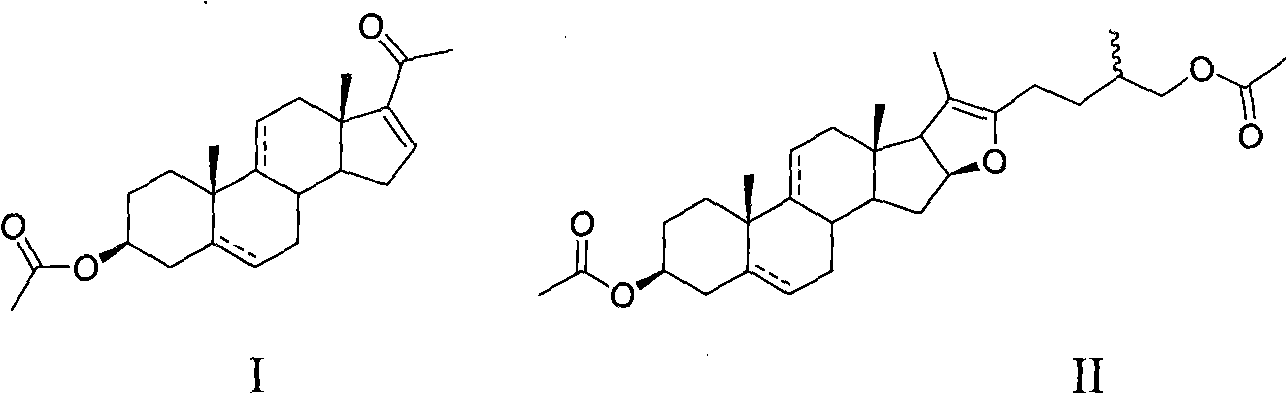
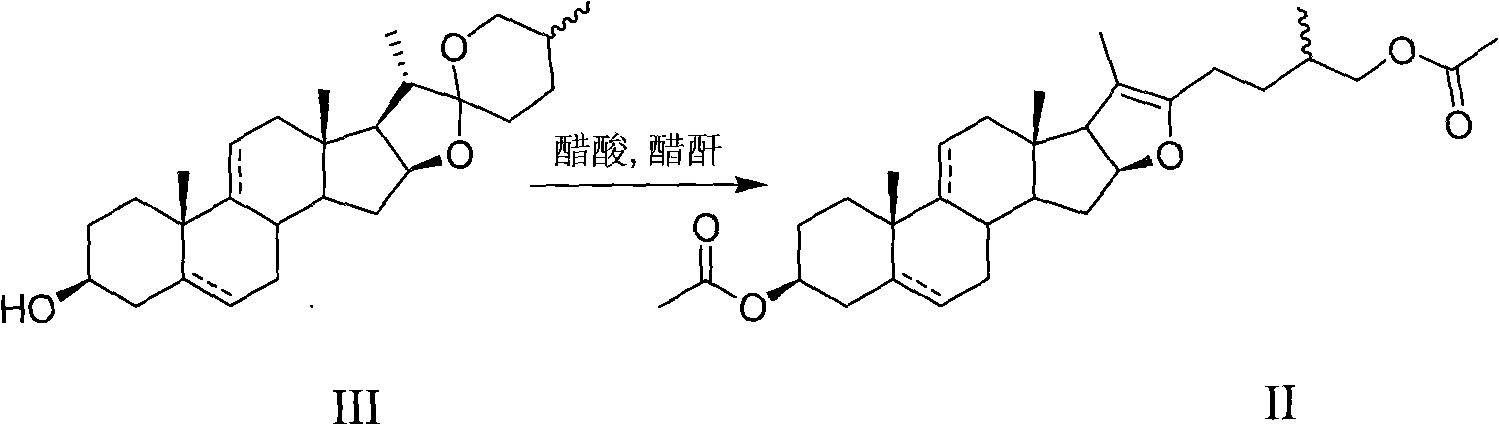
Preparation method of 16-dehydropregnenolone acetate and 16-dehydropregnenolone acetate congeners
A technology of gestational dienolone acetate and its congeners is applied in the field of green preparation of gestational dienolone acetate and its congeners, can solve the problems of complex production process, pollute the environment, increase costs and the like, achieve high yield, The effect of reducing production and labor costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Oxidation of pseudodiosgenin acetate to gestational dienolone acetate:
[0032] Put 50 g of diosgenin in a pressurized kettle, add acetic anhydride and acetic acid to dissolve, heat for 1 hour, recover acetic acid and acetic anhydride under reduced pressure, and recrystallize the obtained crude pseudodiosgenin acetate with methanol to obtain pseudodiosgenin B Pure esters.
[0033] In the reaction bottle, suspend 10g (20mmol) pseudodiosgenin acetate in 15mL water, add 10g tetrafluoroethylene particles (particle size 0.5-5cm), 1.2g (3mmol) methyl trioctyl ammonium chloride, 0.15 g(0.8mmol)V 2 o 5 , 5.7g (50mmol) hydrogen peroxide (30%H 2 o 2 ), mechanically stirred in an oil bath at 85°C for 5.5 hours. Pour out the water layer and keep it for the next recycling. The residue is continuously extracted with petroleum ether until there is no product in the chromatographic detection. The remaining residue is reserved for the next recycling. Concentrate the extract to reco...
Embodiment 2
[0035] Recycle catalyst oxidation pseudodiosgenin acetate to become the reaction system of gestational dienolone acetate (connected to Example 1):
[0036] Add the reclaimed water layer and crystallization mother liquor recovery to the residual system after petroleum ether extraction, then add 10g (20mmol) pseudodiosgenin acetate, 5.7g (50mmol) hydrogen peroxide (30%H 2 o 2 ), mechanically stirred in an oil bath at 85°C for 5.5 hours. Pour out the water layer (reserved for next recycling), and the residue is continuously extracted several times with petroleum ether until no product is detected by chromatography (residue is reserved for next recycling), concentrated and recovered petroleum ether (reserved for next recycling), and then purified with methanol Recrystallization (methanol and its residues recovered from the mother liquor are reserved for the next cycle) to obtain acetic acid pregnant dienolone, and the product characterization data are the same as in Example 1. T...
Embodiment 3
[0038] Oxidation of sisal-genin acetate to 3β-acetoxy-5α-pregna-16(17)-en-20-one:
[0039] Put 50g of sisal sapogenin in a pressurized kettle, add acetic anhydride and acetic acid to dissolve, heat for 1 hour, recover acetic acid and acetic anhydride under reduced pressure, and recrystallize the obtained crude pseudo sisal sapogenin acetate with methanol to obtain pseudo sisal Pure saponin acetate.
[0040] In a reaction flask, suspend 10g (20mmol) of pseudosisalin acetate in 50mL of water, add 10g of tetrafluoroethylene particles, 1.2g (3mmol) of methyl trioctyl ammonium chloride, 0.15g (0.8mmol) of V 2 o 5 , 5.7g (50mmol) hydrogen peroxide (30%H 2 o 2 ), mechanically stirred in an oil bath at 85°C for 5.5 hours. Pour out the water layer (reserve for next cycle use), the residue is continuously extracted several times with normal hexane until no product is detected by chromatography (residue is reserved for next cycle use), concentrated crystallization (n-hexane and its r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com