Multiple thiophene group-containing photochromic compound
A photochromic and compound technology, applied in the fields of silicon organic compounds, color-changing fluorescent materials, organic chemistry, etc., can solve the problems of difficult optical information storage, difficult preparation of mixed crystal materials, and difficulty in mass production, and achieve good dissolution. high stability, easy bridging and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
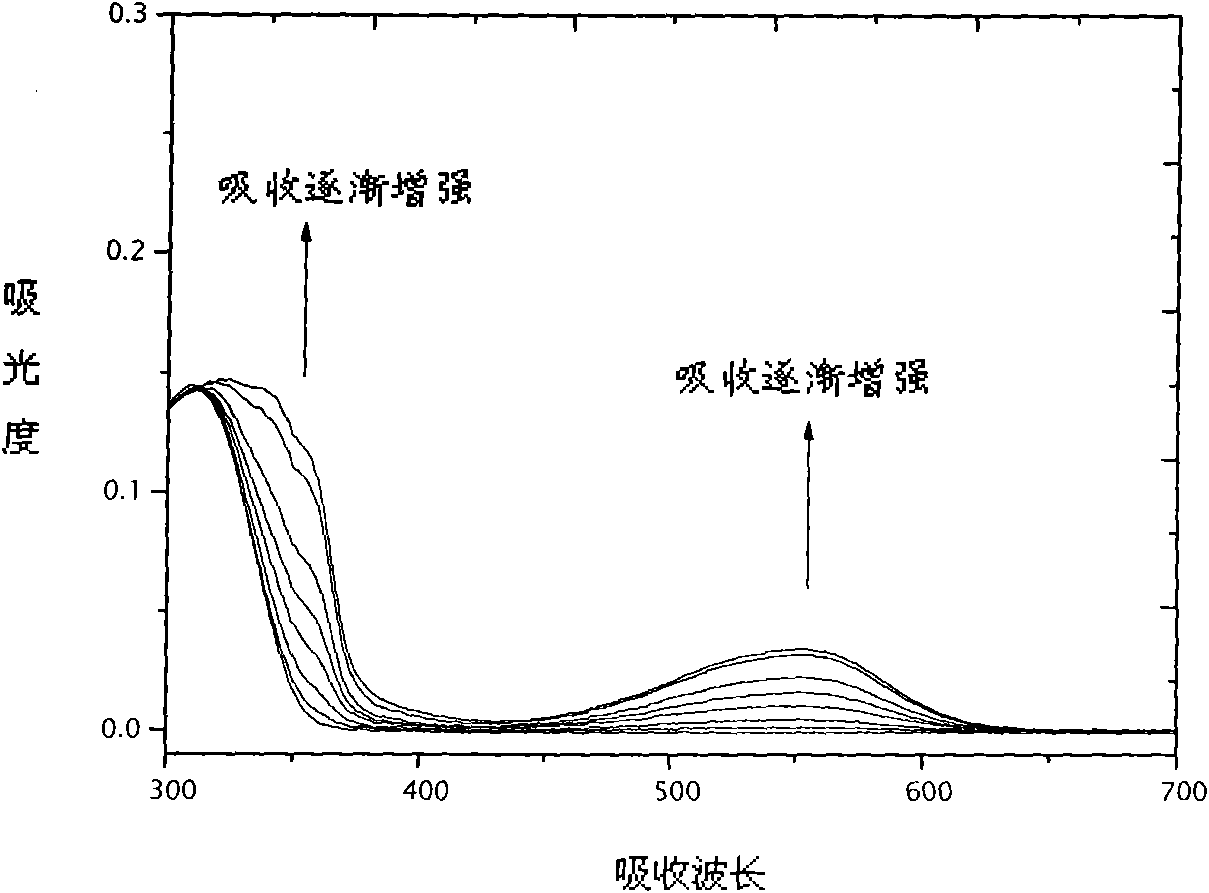
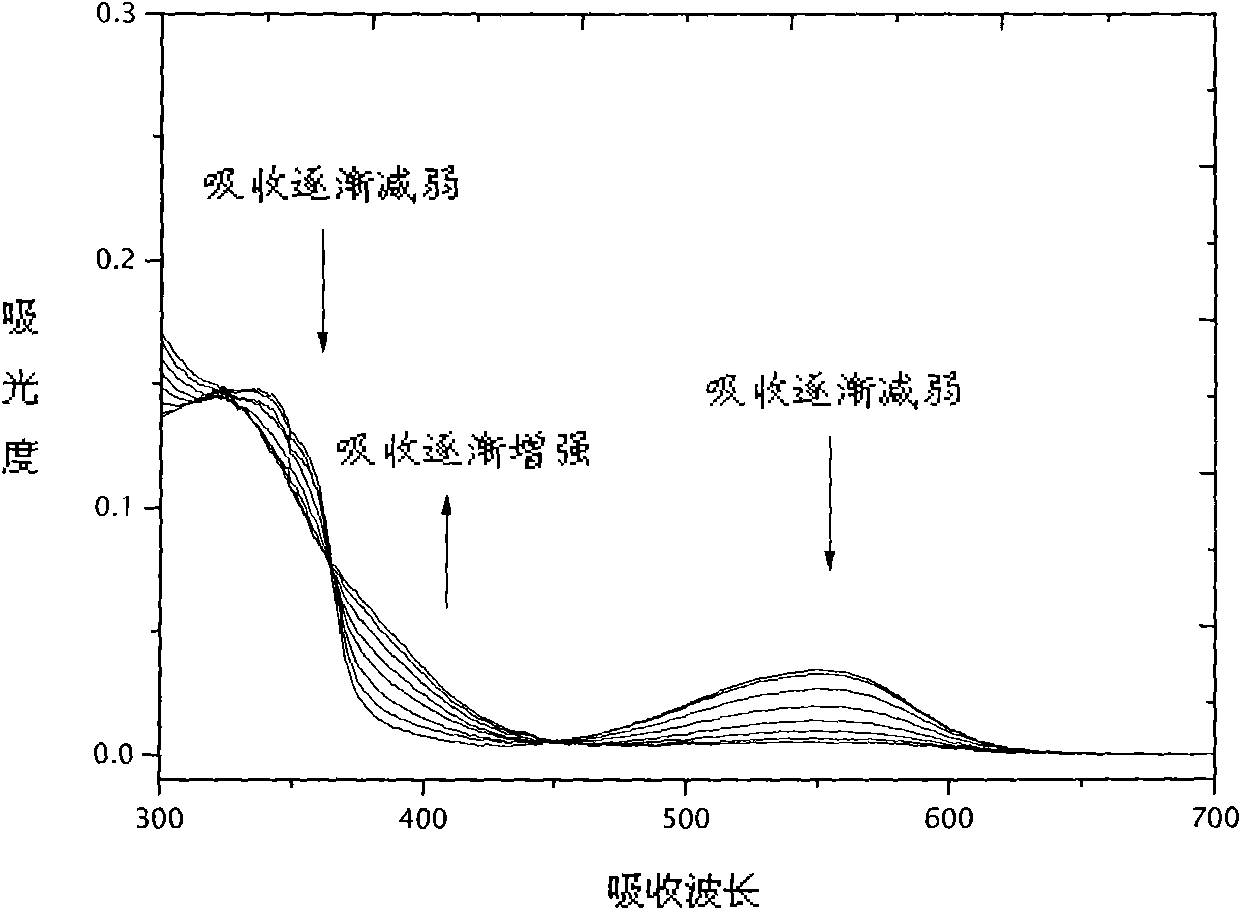
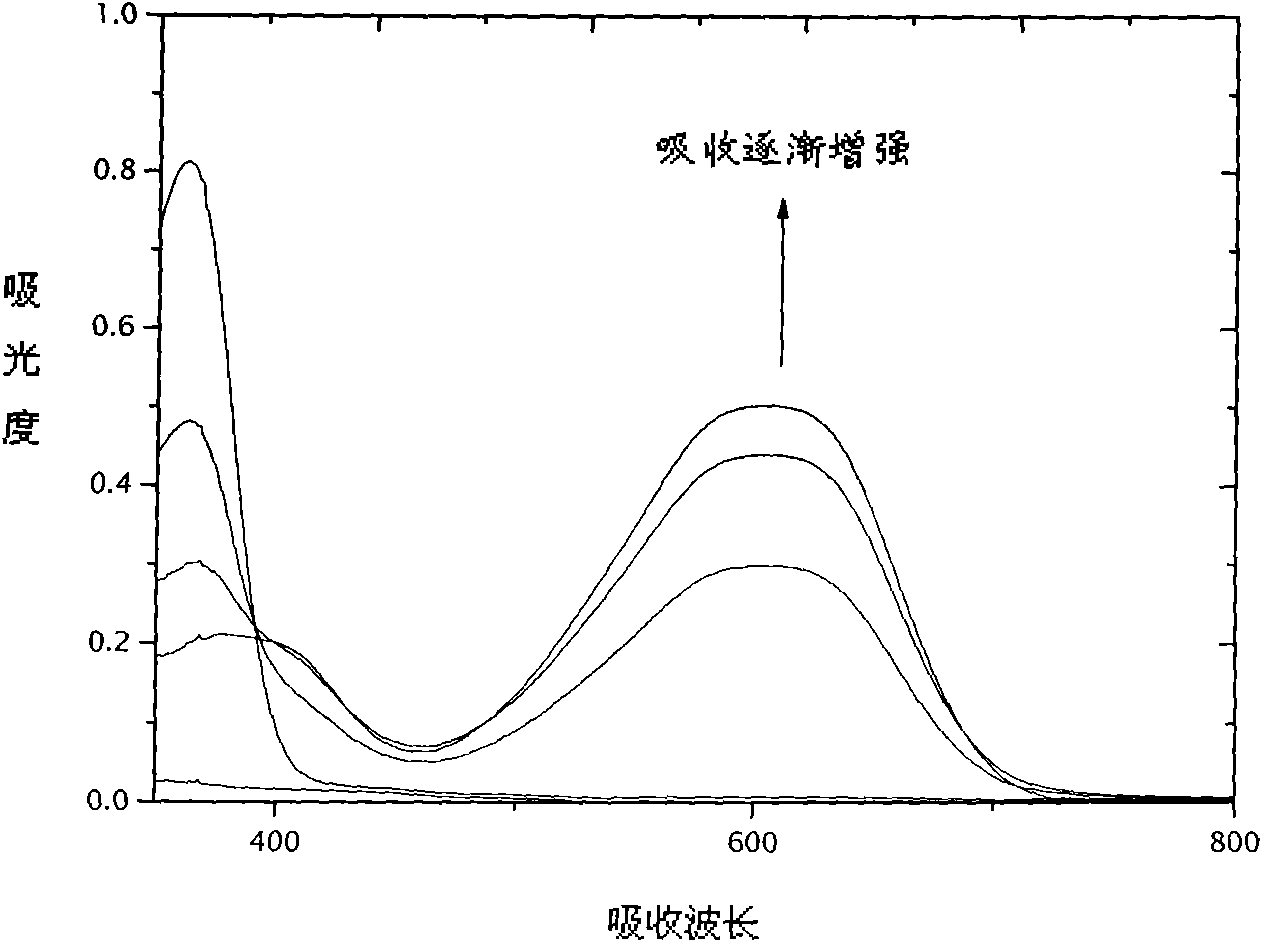
Image
Examples
Embodiment 1
[0043] Preparation of 2,3,4,5-tetrakis(2',5'-dimethyl-3'-thiophene) basethiophene (compound I-1)
[0044]
[0045] In the 50ml two-necked bottle, add tetrabromothiophene 1mmol (compound 3), Ni(dppp)Cl successively 2 and THF, evacuated, Ar 2 Gas displacement, then dropwise thereinto 2,5-dimethyl-3-thiophene magnesium bromide (compound 2, it is made of 5mmol 2,5-dimethyl-3-bromothiophene (compound 1) with 6mmol magnesium chips ), and heated to 80°C for 12 hours under dark conditions. Cool to room temperature, spin off the solvent, and separate by silica gel column chromatography (petroleum ether) to obtain a light yellow solid product. Yield 33%. 1 H NMR (400MHz, CDCl 3 ): δ2.04(s, 3H, CH 3 ), 2.06(s, 3H, CH 3 ), 2.10 (s, 3H, CH 3 ), 2.34 (s, 3H, CH 3 ), 2.37 (s, 6H, CH 3 ), 2.41 (s, 3H, CH 3 ), 2.54 (s, 3H, CH 3 ), 6.45(s, 1H, thiophene-H), 6.50(s, 1H, thiophene-H), 6.81(s, 1H, thiophene-H), 6.99(s, 1H, thiophene-H).MS(ESI) calcd for C 28 h 28 S 5 : 524.08; Fo...
Embodiment 2
[0047] Preparation of 2,3,4,5-tetrakis(2',5'-dimethyl-3'-thiophene) basethiophene (compound I-1)
[0048]
[0049] In a 50ml two-necked flask, add 2.5mmol 2,5-dimethyl-3-thiophene boronic acid (compound 4), tetrabromothiophene (0.2g, 0.5mmol), tetrakis(triphenylphosphine) palladium (0.173g, 0.15mmol ), 1ml water, potassium carbonate (0.276g), 30ml toluene and 3ml ethanol, vacuum, Ar 2 Gas replacement, protected from light, heated to 80 ° C for 48 hours. Cool to room temperature, separate the layers, extract the aqueous phase with dichloromethane, combine the organic phases, spin off the solvent, and separate by silica gel column chromatography (petroleum ether) to obtain a yellow solid. Yield 40%. The structural characterization data are the same as in Example 1.
Embodiment 3
[0051] Preparation of 3,4-two (5'-methyl-2'-thiophene) base-2,5-bis(2',5'-dimethyl-3'-thiophene) base thiophene (compound I-2)
[0052]
[0053] 0.5 mmol of 3,4-bis(5'-methyl-2'-thiophene)-2,5-dibromothiophene (compound 5) and catalyst (Ni(dppp)Cl 2 ) were added together in 30 mL of diethyl ether, and a diethyl ether solution of a metal organic reagent (compound 2) prepared from 1.5 mmol of 2,5-dimethyl-3-bromothiophene and metal powder was added dropwise. After the dropwise addition was completed, the reaction was carried out overnight. Dilute hydrochloric acid was poured into the reaction mixture to terminate the reaction. The organic phase was separated and the aqueous phase was extracted three times with ether. The organic phases were combined and washed with water. After drying over anhydrous magnesium sulfate, diethyl ether was removed by rotary evaporation. The residue was separated by silica gel column (using petroleum ether as eluent) to obtain compound I-2 wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com