Enterococcus faecium ANSE228 and application thereof
A technology of Enterococcus faecalis and seeds, applied to Enterococcus faecalis and its application fields, can solve the problems of increasing the probability of occurrence of digestive tract diseases of livestock and poultry, mutating pathogenic bacteria into drug-resistant strains, destroying the microecological balance of animals, etc., and achieving significant probiotics. performance, reduce production costs, and improve production performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of embodiment 1 enterococcus faecalis ANSE228 fermented liquid
[0040] Get Enterococcus faecalis ANSE228 (preservation number is CGMCC No.4082, viable bacteria concentration is 10 9 CFU / ml), inoculated in 50ml medium for shake flask fermentation culture, the fermentation temperature was 37°C, the pH value was 7.0, static culture, and the fermentation time was 24h.
[0041] Among them, the shake flask fermentation medium is composed of the following components: corn flour 15g, soybean meal 20g, glucose 5g, dipotassium hydrogen phosphate 0.15g, potassium dihydrogen phosphate 1.5g, manganese sulfate monohydrate 1.0g, magnesium sulfate heptahydrate 1.5 g, distilled water 1000mL, pH value is 7.0.
[0042] After the shake flask fermentation, carry out the fermenter pilot test, take 50ml of shake flask fermentation seed liquid and inoculate it into a 10L fermenter, the liquid volume is 5L, the fermentation temperature is 37°C, the pH value is 7.0, the stirrin...
Embodiment 2
[0044] Example 2 Probiotic verification of Enterococcus faecalis ANSE228
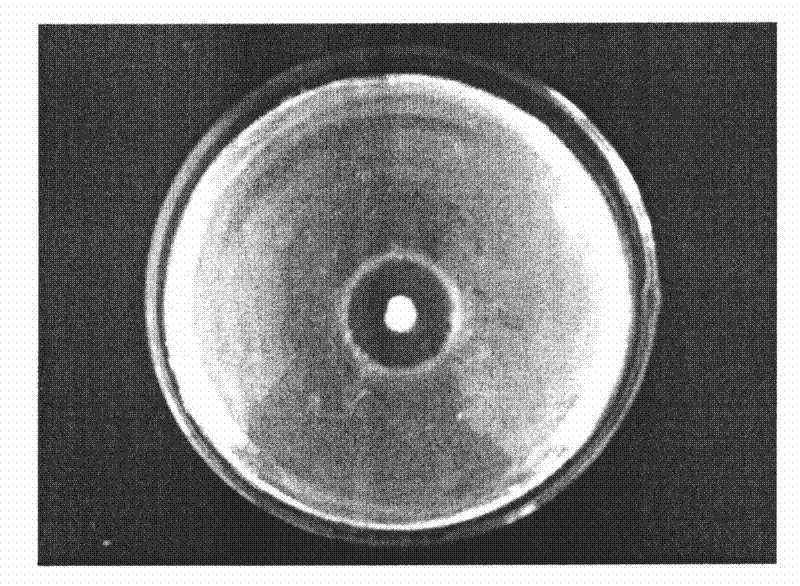
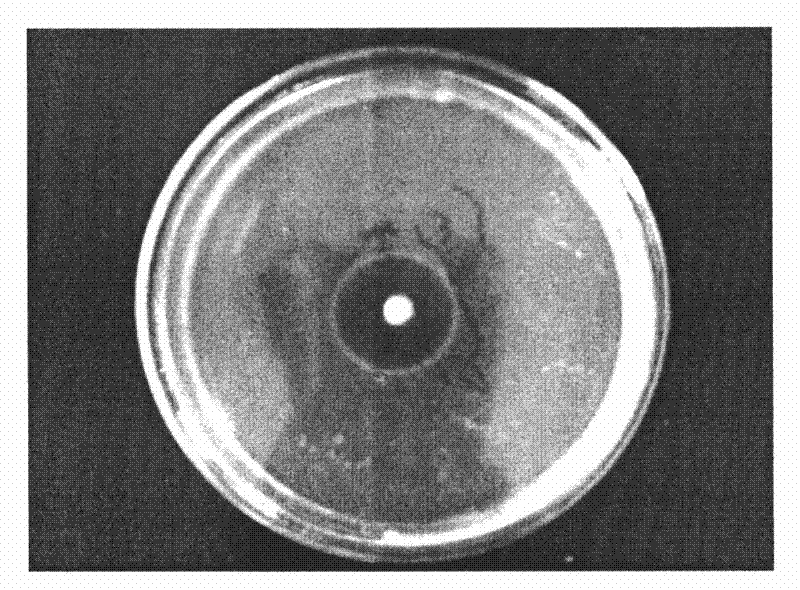
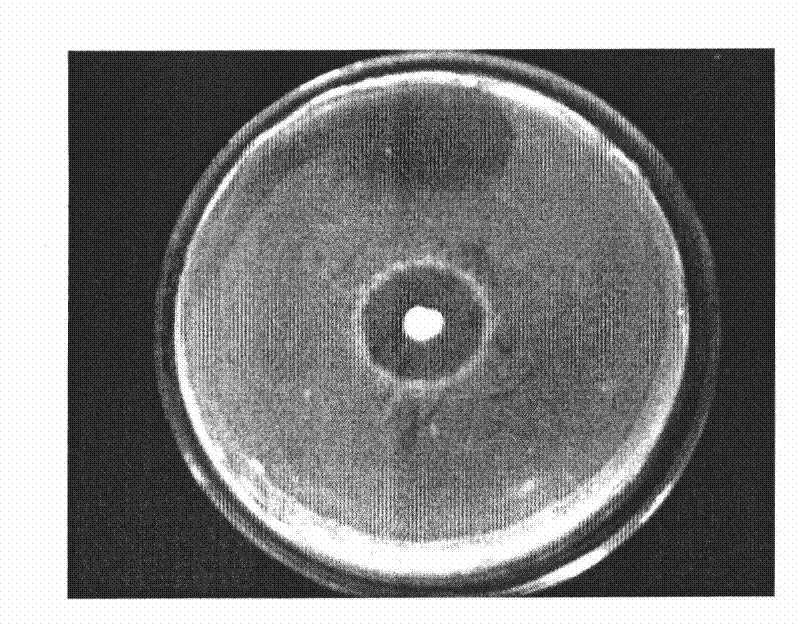
[0045] Prepare an MRS plate with a thickness of about 4mm, and make the concentration of pathogenic bacteria (Escherichia coli, Staphylococcus aureus and Salmonella) 10 6 Spread 100 μL of the bacteria suspension on the plate, and place a sterilized stainless steel small tube (round small tube with an inner diameter of 6 mm, an outer diameter of 8 mm, and a height of 10 mm, with smooth ends) placed on the medium by aseptic operation. , gently pressurized so that it contacts with the culture medium without gaps, and after a few minutes, add 200-300 microliters of the fermented liquid (activated strain bacterial liquid) prepared in Example 1 dropwise to each small tube respectively. Make it overflow, incubate at 37°C for 24 hours, and then measure the diameter of the inhibition zone. Each experiment was repeated three times and the average value was taken. The result is as Figure 1-3 shown.
[0046] W...
Embodiment 3
[0048] Example 3 Stress resistance verification of Enterococcus faecalis ANSE228
[0049] 1. Take the preserved 5mL fermented liquid prepared in Example 1 and pour it into the centrifuge tube 1, use ten-fold serial dilution, and spread it on the MRS plate 1. Then put the centrifuge tube 2 containing 5mL of the fermented liquid prepared in Example 1 in a water bath at 80°C and heat it for 15 minutes, take the heated Enterococcus faecalis bacterial liquid and carry out ten-fold stepwise dilution, and spread it on the MRS plate 2 . Finally, the plates before and after heating were cultured at 37° C. for 24 hours, and the number of Enterococcus faecalis ANSE228 before and after heating was calculated.
[0050] The results showed that the survival rate of live bacteria reached 85%.
[0051] 2. Tolerance test of simulated gastric juice: Dissolve pepsin in 0.5% normal saline to make the final concentration 3g / L, and adjust the pH value to 2.0 with concentrated hydrochloric acid or ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com