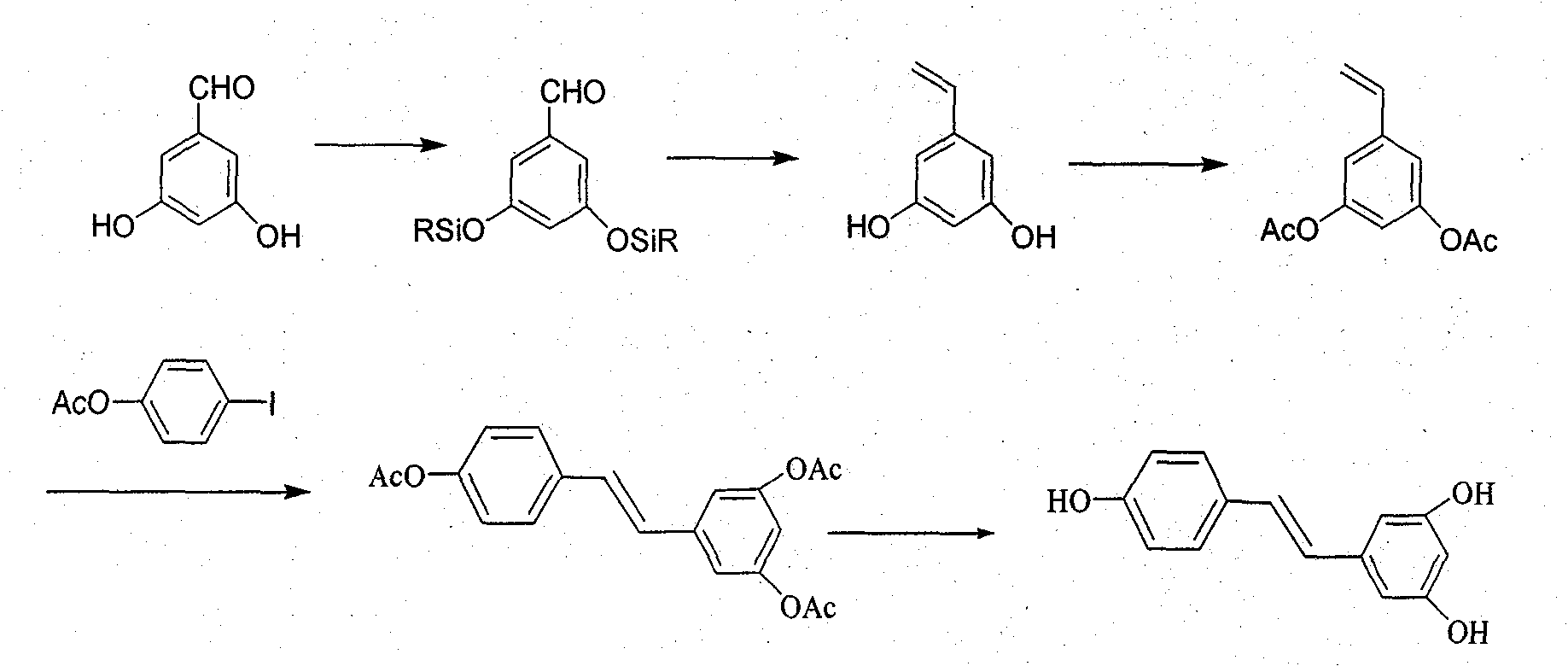
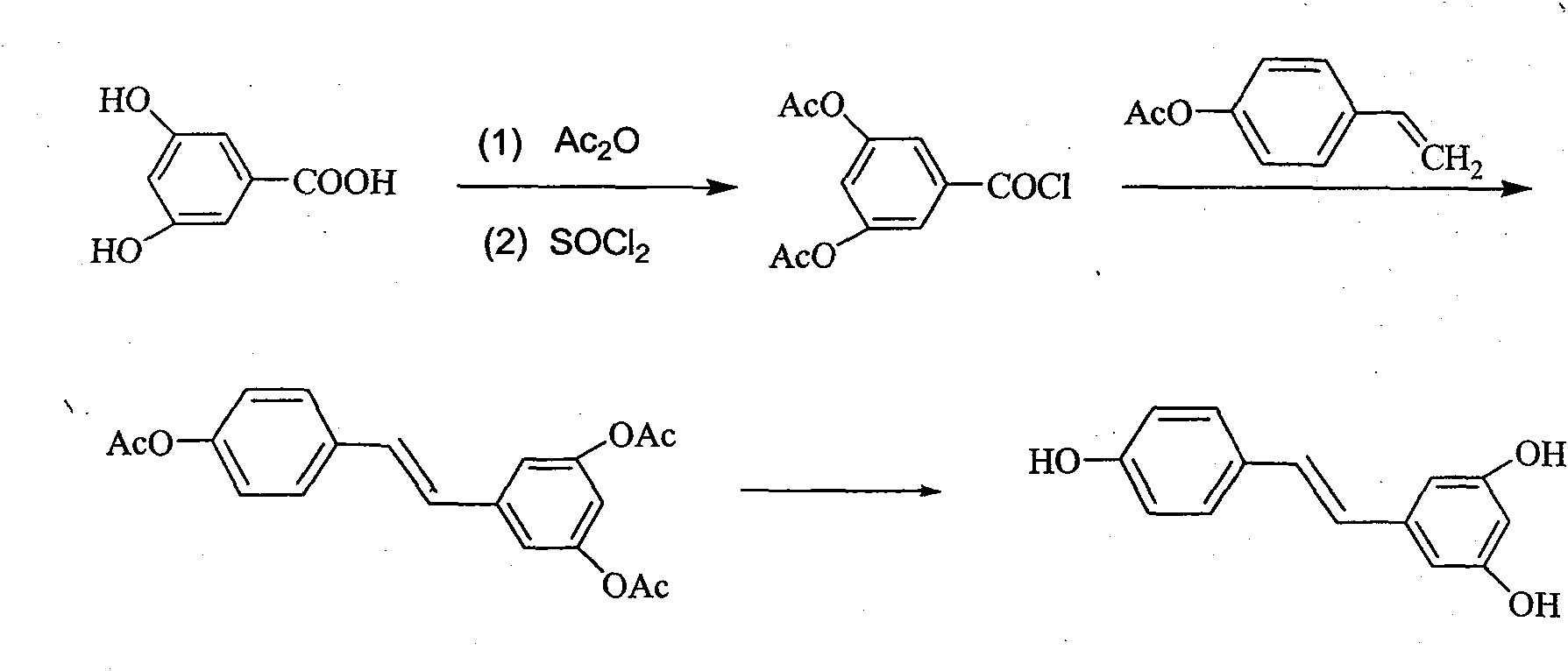
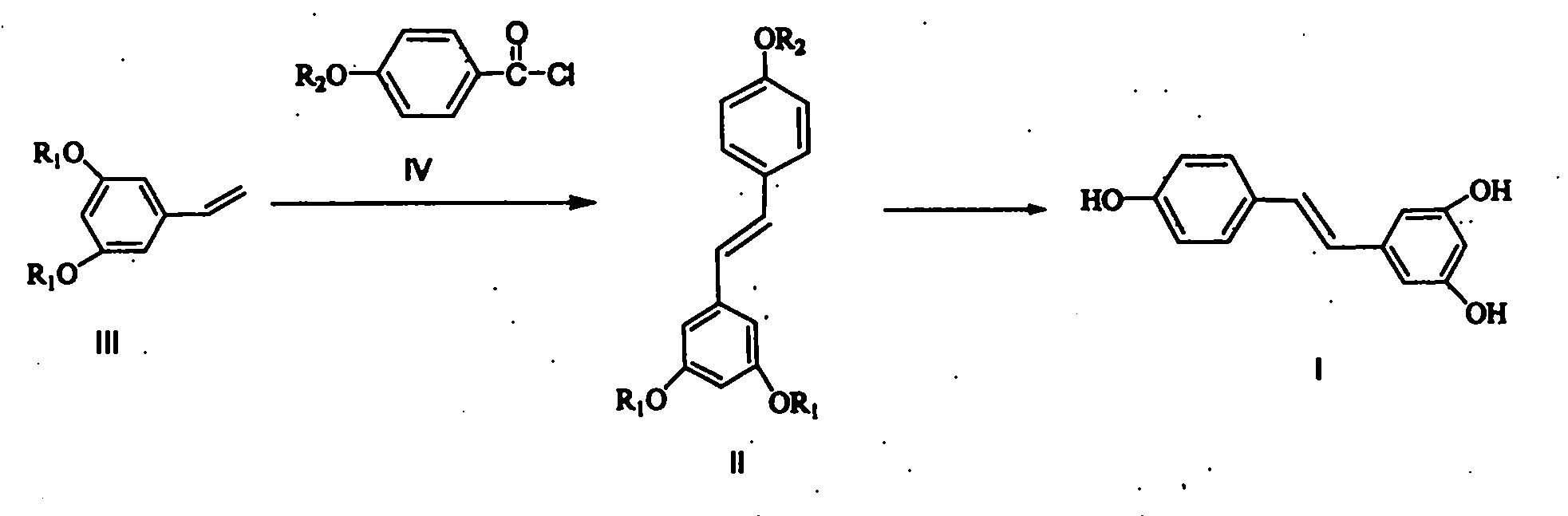
Novel method for preparing resveratrol and derivative thereof through decarbonylation heck reaction
A technology of resveratrol and derivatives, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problem of high cost and unfavorable preparation of three-substituted resveratrol resveratrol , difficult to obtain and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] 1.1 3,5-dibenzyloxy-4 Synthesis of ′-methoxystilbene
[0046] In a 1000ml reaction flask, add 200ml of p-xylene and 20ml of N-methylmorpholine, then 31.6g of 3,5-dibenzyloxystyrene and 18.0g of p-methoxybenzoyl chloride, then add 0.15g of palladium acetate and 0.3g of N,N'-bis(2,6-diisopropylphenyl)imidazolium chloride, stirred well, raised the temperature to 120°C, and reacted for 4h under the protection of nitrogen. After the reaction was completed, cool to room temperature, filter, and the resulting filtrate was washed twice with 300ml of 5% sodium carbonate solution, then the organic layer was dried with anhydrous magnesium sulfate, filtered, concentrated to recover the solvent, and then recrystallized by adding 100ml of ethanol to obtain 34.2g 3,5-dibenzyloxy-4 '-Methoxystilbene, melting point 94-96°C, yield 81%.
[0047] 1.2 Preparation of 3,4′,5-trihydroxystilbene (resveratrol)
[0048] Take 21.1g 3,5-dibenzyloxy-4 '-Methoxystilbene, be dissolv...
Embodiment 2
[0050]
[0051] 2.1 3,-4 ', Synthesis of 5-trimethoxystilbene
[0052] In a 1000ml reaction flask, add 150ml of p-xylene and 20ml of N-methylmorpholine, then 16.4g of 3,5-dimethoxystyrene and 19.0g of p-methoxybenzoyl chloride, then add 0.15g of palladium acetate and 0.3g of N,N'-bis(2,6-diisopropylphenyl)imidazolium chloride, stirred well, raised the temperature to 100°C, and reacted for 4h under the protection of nitrogen. After completion of the reaction, cool to room temperature, filter, and the resulting filtrate is washed twice with 200ml of 5% sodium carbonate solution, then the organic layer is dried with anhydrous magnesium sulfate, filtered, concentrated to recover the solvent, and then recrystallized by adding 40ml of acetone to obtain 21.0g 3,-4 ', 5-trimethoxystilbene, melting point 56-57°C, yield 78%.
[0053] 2.2 Preparation of 3,4′,5-trihydroxystilbene (resveratrol)
[0054] Other conditions are the same as example 1.2, will 3,5-dibenzyloxy-4 '-Methoxys...
Embodiment 3
[0056]
[0057] 3.1 Synthesis of 3,5-dimethoxy-4′-acetoxystilbene
[0058] In a 1000ml reaction flask, add 150ml of p-xylene and 20ml of N-methylmorpholine, then 16.4g of 3,5-dimethoxystyrene and 22.0g of p-acetoxybenzoyl chloride, then add 0.15g of palladium acetate and 0.3 g N,N'-bis(2,6-diisopropylphenyl)imidazolium chloride, stir well, raise the temperature to 120-130°C, and react for 12h under the protection of nitrogen. After the reaction was completed, cool to room temperature, filter, and the resulting filtrate was washed twice with 200ml of 5% sodium carbonate solution, then the organic layer was dried with anhydrous magnesium sulfate, filtered, concentrated to recover the solvent, and then recrystallized by adding 20% ethanol water to obtain 24.4 g 3,5-dimethoxy-4'-acetoxystilbene, yield 82%.
[0059] 3.2 Synthesis of 3,5-dimethoxy-4′-hydroxystilbene
[0060] 15g of 3,5-dimethoxy-4'-acetoxystilbene was dissolved in 80ml of tetrahydrofuran, 30ml of water and 40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com