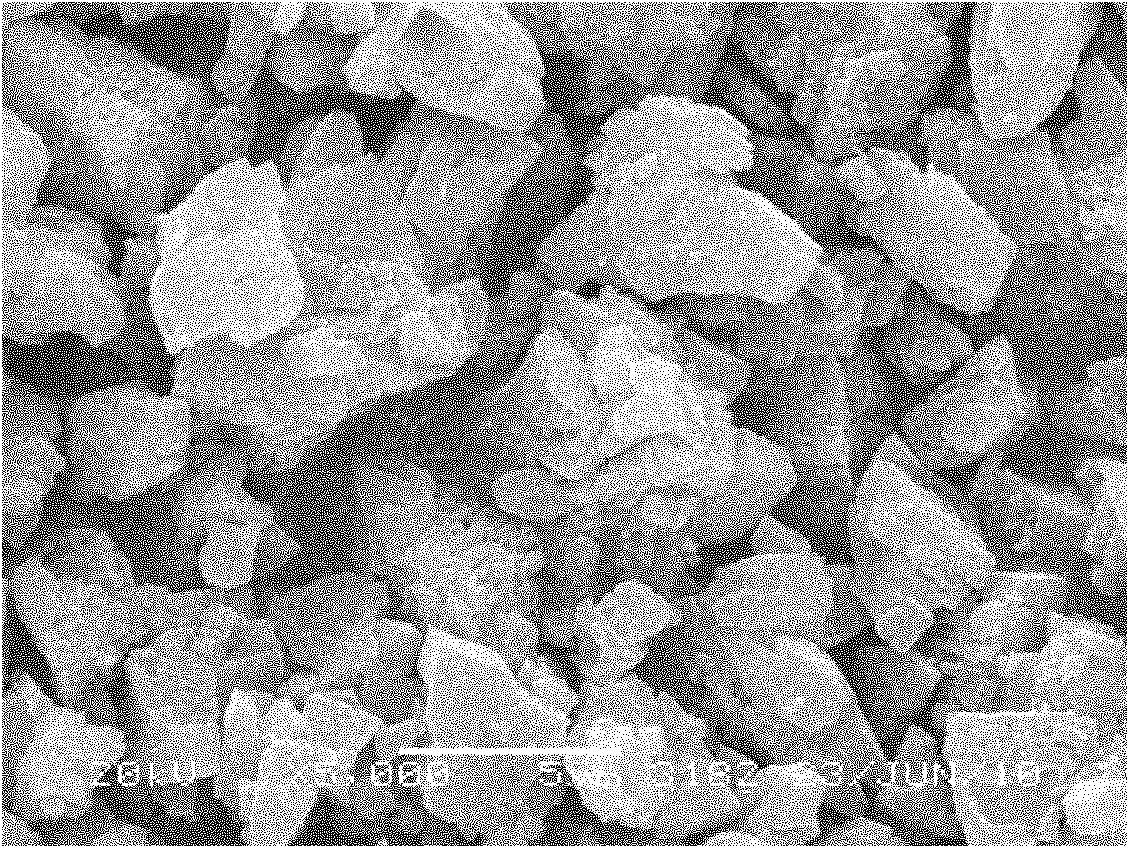Method for preparing phosphate series lithium ion battery anode material
A technology for lithium-ion batteries and positive electrode materials, which is applied in battery electrodes, circuits, electrical components, etc., and can solve the problem of poor performance of positive electrode materials of one-element or multi-element composite phosphate lithium-ion batteries, and uniform particle size distribution of precursors and precipitants. Mix unevenly with salt solution and other problems to achieve the effect of avoiding uneven mixing, good batch performance and good consistency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
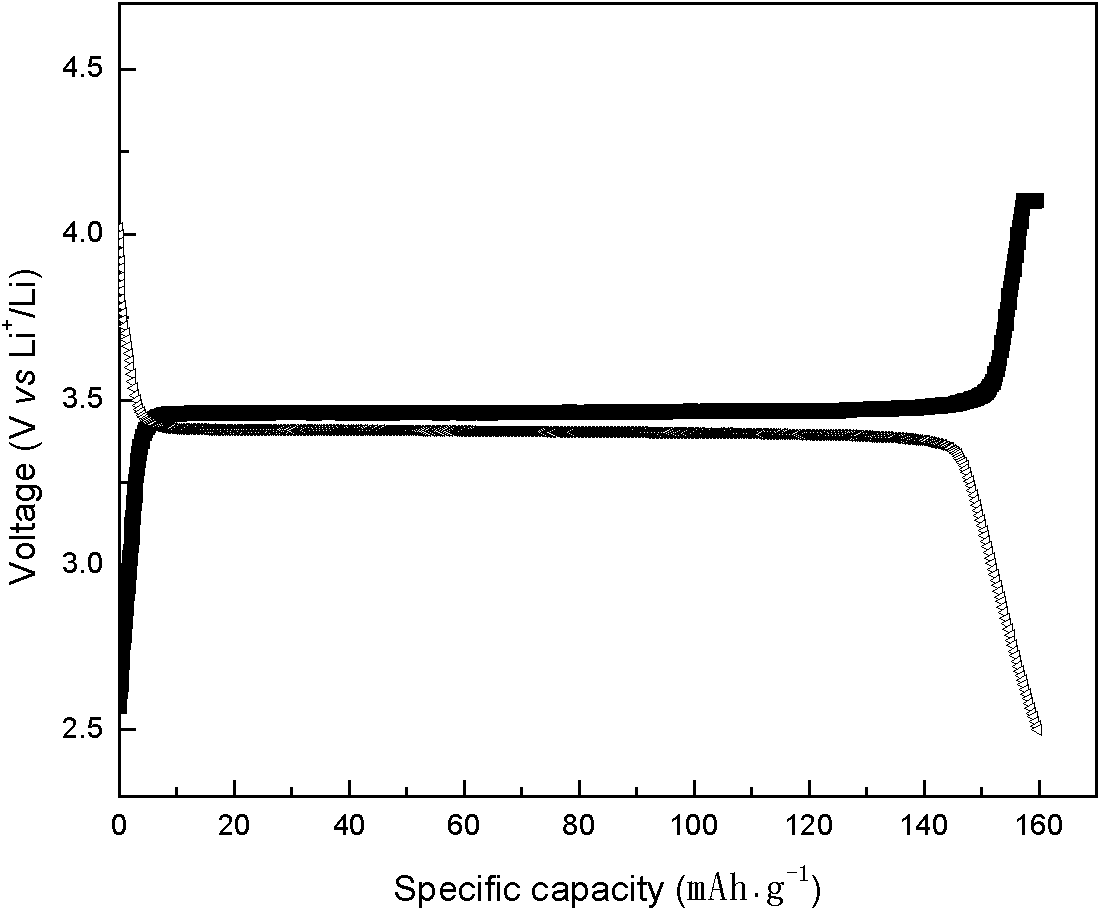
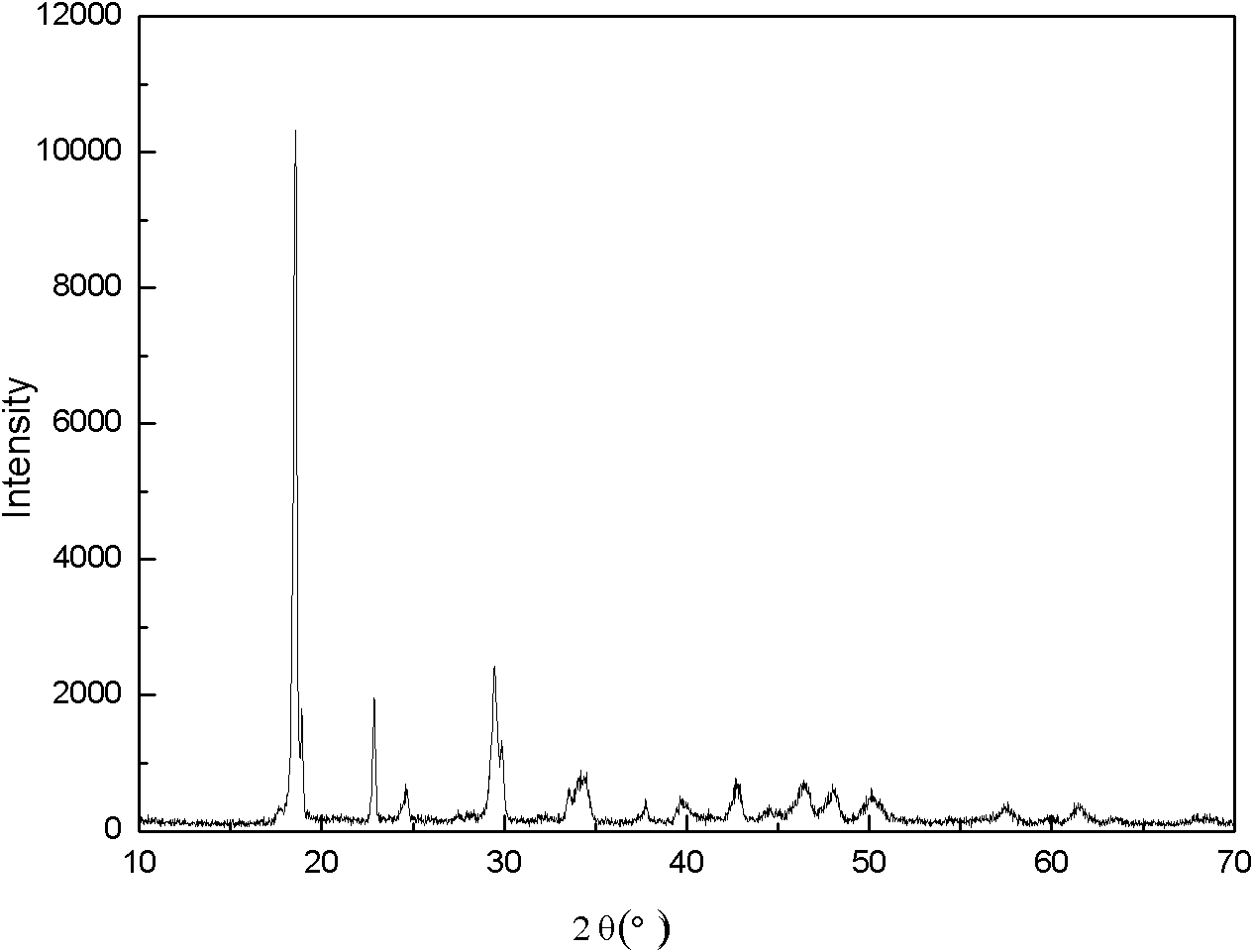
[0028] Prepare ferrous sulfate aqueous solution, concentration is 2.0 mol / liter, every liter of solution adds 0.5 mole of sulfuric acid and 100 grams of urea, adds precipitating agent oxalic acid and total metal ion molar ratio is 1.1: 1, stirs and dissolves solid to make system into solution, passes The heating mantle heats the solution and controls the reaction time. With the decomposition of urea, the hydrogen ions in the solution are consumed, the pH value of the solution increases gradually, and the solubility of the precipitate gradually decreases and precipitates. The temperature in the reactor is 120°C, the reaction is stirred for 14 hours, filtered and washed until the sulfate radical in the washing water cannot be detected with barium chloride, and then dried to obtain FeC 2 o 4 2H 2 O unary precursor. The precursor was added stoichiometric ratio of ammonium dihydrogen phosphate and lithium hydroxide, and the sample was obtained by ball milling and drying in aceton...
Embodiment 2
[0030]Prepare manganese sulfate, ferrous sulfate, cobalt sulfate mixed aqueous solution, wherein the total concentration of manganese sulfate, ferrous sulfate, cobalt sulfate is 1.5 mol / liter, the molar ratio of the three is 5:4:1, every liter of solution is added 2 moles nitric acid and 80 grams of urea, adding precipitant ammonium oxalate and the total metal ion molar ratio is 3: 1, stirring and dissolving the solid to make the system into a solution, heating the solution through a heating mantle, controlling the reaction time, along with the decomposition of urea, the solution will The hydrogen ions are consumed, the pH value of the solution increases gradually, and the solubility of the precipitate decreases gradually. The temperature inside the reactor was controlled to be 90°C. The reaction was stirred for 12 hours, filtered and washed until the sulfate radical in the washing water could not be detected with barium chloride, and dried to obtain Mn 0.5 Fe 0.4 co 0.1 C ...
Embodiment 3
[0032] Prepare manganese sulfate, ferrous sulfate, nickel sulfate, cobalt sulfate mixed aqueous solution, wherein the total concentration of manganese sulfate, ferrous sulfate, nickel sulfate, cobalt sulfate is 2 mol / liter, and the mol ratio of the four is 1: 1: 1: 1. Add 3 moles of hydrochloric acid and 60 grams of urea per liter of solution, add the precipitant oxalic acid and the total metal ion molar ratio is 2:1, stir and dissolve the solid to make the system into a solution, heat the solution through the heating mantle, control the reaction time, and With the decomposition of urea, the hydrogen ions in the solution are consumed, the pH value of the solution increases gradually, and the solubility of the precipitate gradually decreases and precipitates. The temperature inside the reactor was controlled to be 150°C. The reaction was stirred for 10 hours, filtered and washed until the sulfate radical in the washing water could not be detected with barium chloride, and dried...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com