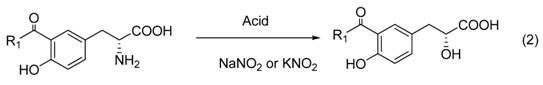
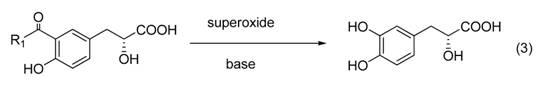Method for synthesizing tanshinol
A synthesis method and technology of danshensu are applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate and other directions, which can solve the problems of singleness and lack of optical activity, and achieve the effects of easy availability of raw materials and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
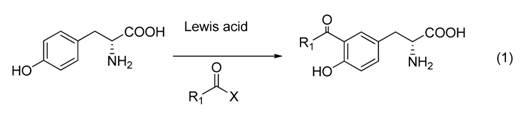
[0030] ( R )-3-(3-acetyl-4-hydroxyphenyl)-2-alanine synthesis:
[0031] Take a 250mL three-neck flask, add 175 mL of nitrobenzene to it, add (7.2 g, 40 mmol) of D-tyrosine at room temperature, then add aluminum trichloride, and add acetyl chloride to the reaction system at one time after stirring (57.3 mmol), the reaction system was gradually heated to 85-90 ° C, reacted for 4-8 h, then poured the mixed solution into the mixed solution of concentrated HCl and ice, removed the organic layer, kept the water layer, and washed the water layer with ethyl acetate The ester was extracted 3 times (50 mL×3), the aqueous layer was crystallized at low temperature, and the precipitated crystals were recrystallized with 5mol / L to finally obtain the compound ( R )-3-(3-acetyl-4-hydroxyphenyl)-2-alanine, beige crystal. m.p 208-214°C, the melting point of the product is consistent with that of known compounds; MS (ESI) [M+H] + m / z 224.2.
Embodiment 2
[0033] ( R )-3-(3-butyryl-4-hydroxyphenyl)-2-alanine synthesis:
[0034] Take a 250 mL three-neck flask, add 175 mL of nitrobenzene to it, add (7.2 g, 40 mmol) of D-tyrosine at room temperature, then add aluminum trichloride, and add butyryl chloride to the reaction system at one time after stirring (57.3 mmol), the reaction system was gradually heated to 85-90 ° C, reacted for 4-8 h, then poured the mixed solution into the mixed solution of concentrated HCl and ice, removed the organic layer, kept the water layer, and washed the water layer with ethyl acetate The ester was extracted 3 times (50 mL×3), the aqueous layer was crystallized at low temperature, and the precipitated crystals were recrystallized with 5mol / L to finally obtain the compound ( R )-3-(3-butyryl-4-hydroxyphenyl)-2-alanine. (4.6 g, 42% yield), solid. The mass spectrum of the product is consistent with that of known compounds.
Embodiment 3
[0036] ( R )-3-(3-hexanoyl-4-hydroxyphenyl)-2-alanine synthesis:
[0037] Take a 250mL three-necked flask, add 88 mL of nitrobenzene to it, add (3.6 g, 20 mmol) of D-tyrosine at room temperature, then add aluminum trichloride, and add acetyl chloride to the reaction system at one time after stirring (2 mL, 29 mmol), the reaction system was gradually warmed up to 85-90 °C, and reacted for 4-8 h, then poured the mixed solution into the mixed solution of concentrated HCl and ice, removed the organic layer, kept the water layer, and the water layer Extracted 3 times with ethyl acetate (25 mL×3), the aqueous layer was crystallized at low temperature, and the precipitated crystals were recrystallized with 5mol / L to finally obtain the compound ( R )-3-(3-hexanoyl-4-hydroxyphenyl)-2-alanine (2.5 g, 44.8% yield). The mass spectrum of the product is consistent with that of known compounds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com