Green mud crab antibacterial peptide Sphistin and application thereof
A technology of antimicrobial peptides and antibacterial peptides, which is applied in the antimicrobial peptide Sphistin and its application fields, can solve the problems of negative effects of drug resistance of pathogenic bacteria, application limitations, etc., achieve significant antibacterial effect, fast sterilization rate, The effect of broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Preparation of Sphistin, an antimicrobial peptide from Scylla pseudocaveae
[0032] Sphistin is derived from the partial N-terminal sequence of the histone H2A of the mud crab, consisting of 38 amino acids. The specific amino acid sequence is:
[0033] Met Ala Gly Gly Lys Ala Gly Lys Asp Ser Gly Lys Ala Lys Ala Lys
[0034] 1 5 10 15
[0035] Ala Val Ser Arg Ser Ala Arg Ala Gly Leu Gln Phe Pro Val Gly Arg
[0036] 20 25 30
[0037] Ile His Arg His Leu Lys
[0038] 35
[0039] The nucleotide sequence of Sphistin, an antimicrobial peptide from Scylla pseudocaveus, is:
[0040] atggctggtg gcaaggcagg caaggactcc ggcaaggcga aagccaaggc agtatcacgc 60
[0041] tcggccagggg ctggtctgca gtttcctgtc ggtcgtatcc atagacacct gaag 114
[0042] The molecular formula of the antibacterial polypeptide is C 170 h 293 N 61 o 46 S 1 , the molecular weight is 3959.61. The 38 amino acids of the antimicrobial peptide contain 10 positively charged amino acid residues and 1 nega...
Embodiment 2
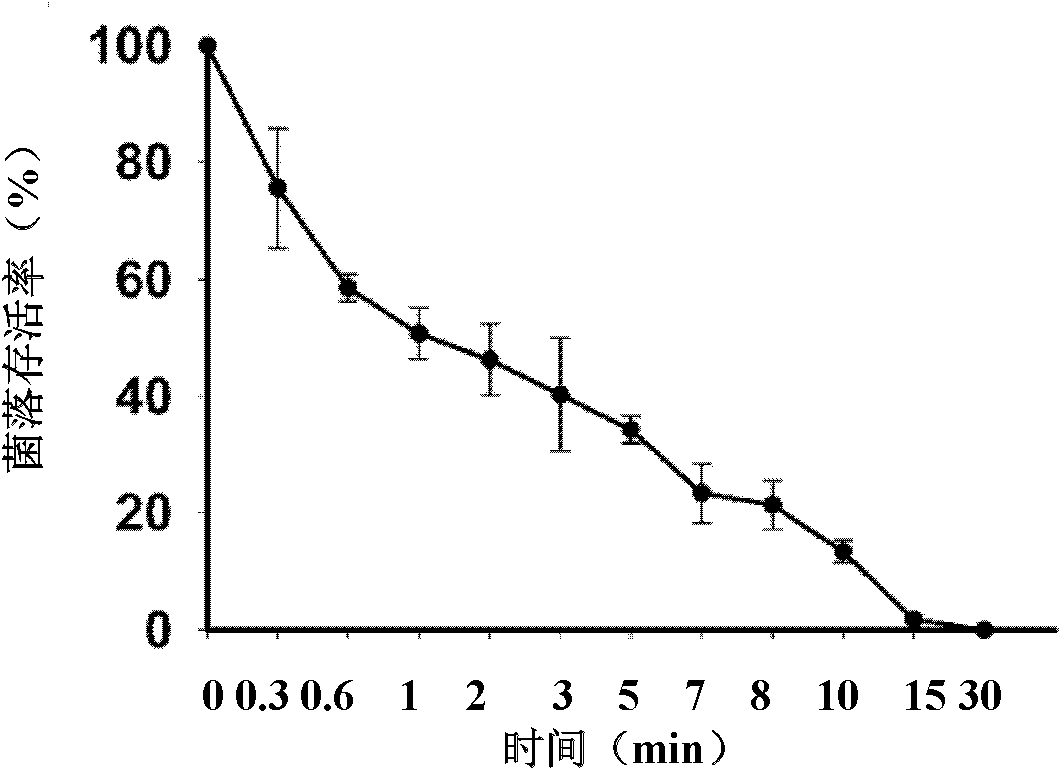
[0044] Example 2 Determination of the minimum inhibitory concentration (MIC: minimum inhibition concentration) of the antimicrobial peptide Sphistin
[0045] (a) The preserved species of Escherichia coli, Escherichia coli MC1061, Staphylococcus epidermidis, Staphylococcus aureus, Corynebacterium glutamicum, Micrococcus lyticus, Bacillus subtilis, Bacillus cereus, Micrococcus luteus, Shiga Bacteria, Pseudomonas stutzeri, and Pseudomonas aeruginosa were streaked on the nutrient broth plate; The temperature was inverted for 12-16 hours; Candida albicans and Pichia pastoris were streaked on the YPG plate, and cultured at 28°C for 1-2 weeks. All the bacteria were inoculated on the corresponding slant, and the culture was continued for 12-16 hours, and the yeast was continued for 3-7 days; the freshly prepared bacterial slant culture was washed with 10mM sodium phosphate buffer (pH 7.4), and the dilution was adjusted to OD 600 =0.003; Vibrio was adjusted and diluted to OD with seaw...
Embodiment 3
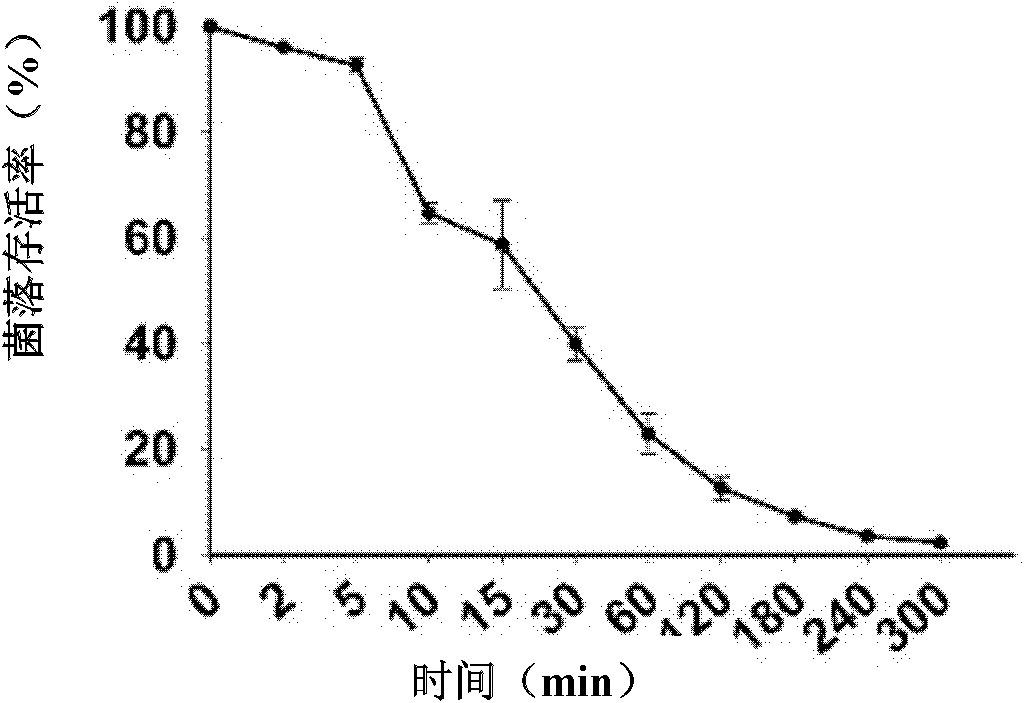
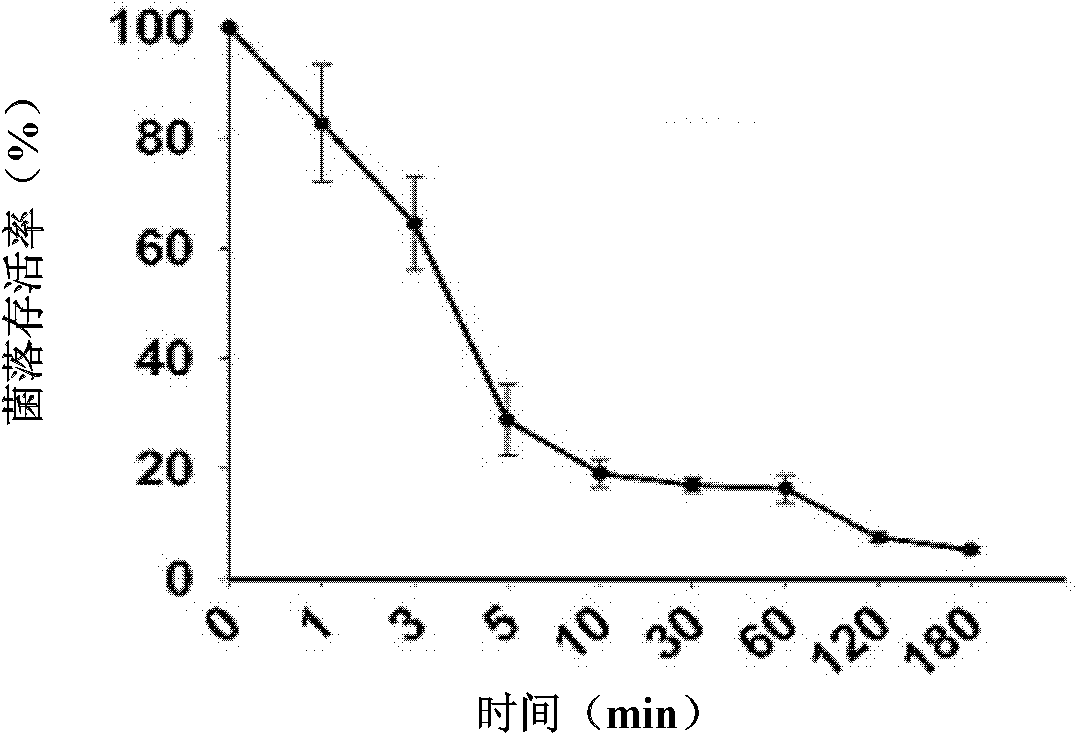
[0062] Example 3 Determination of the minimum bactericidal concentration (MBC: minimum bactericidal concentration) of the antimicrobial peptide Sphistin
[0063] (a) The preserved species of Escherichia coli, Escherichia coli MC1061, Staphylococcus epidermidis, Staphylococcus aureus, Corynebacterium glutamicum, Micrococcus lyticus, Bacillus subtilis, Micrococcus luteus, Shigella, Shigella Pseudomonas, Aeromonas hydrophila, and Pseudomonas fluorescens were streaked on the nutrient broth plate, and cultured upside down at the corresponding temperature for 12-16 hours; Pichia pastoris was streaked on the YPG plate, and cultured at 28°C for one to two weeks. All the bacteria were inoculated on the corresponding slant, and the culture was continued for 12-16 hours, and the yeast was continued for 3-7 days; the freshly prepared bacterial slant culture was washed with 10mM sodium phosphate buffer (pH 7.4), and the dilution was adjusted to OD 600 =0.003; Yeast is adjusted to OD 600 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com