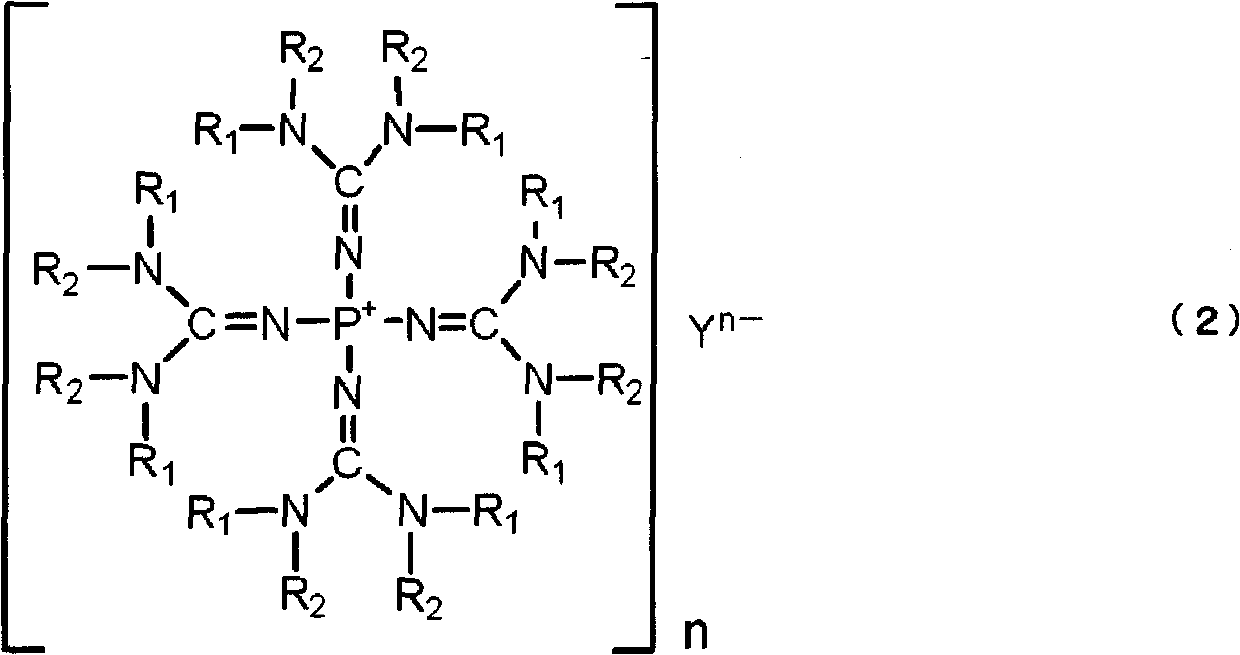
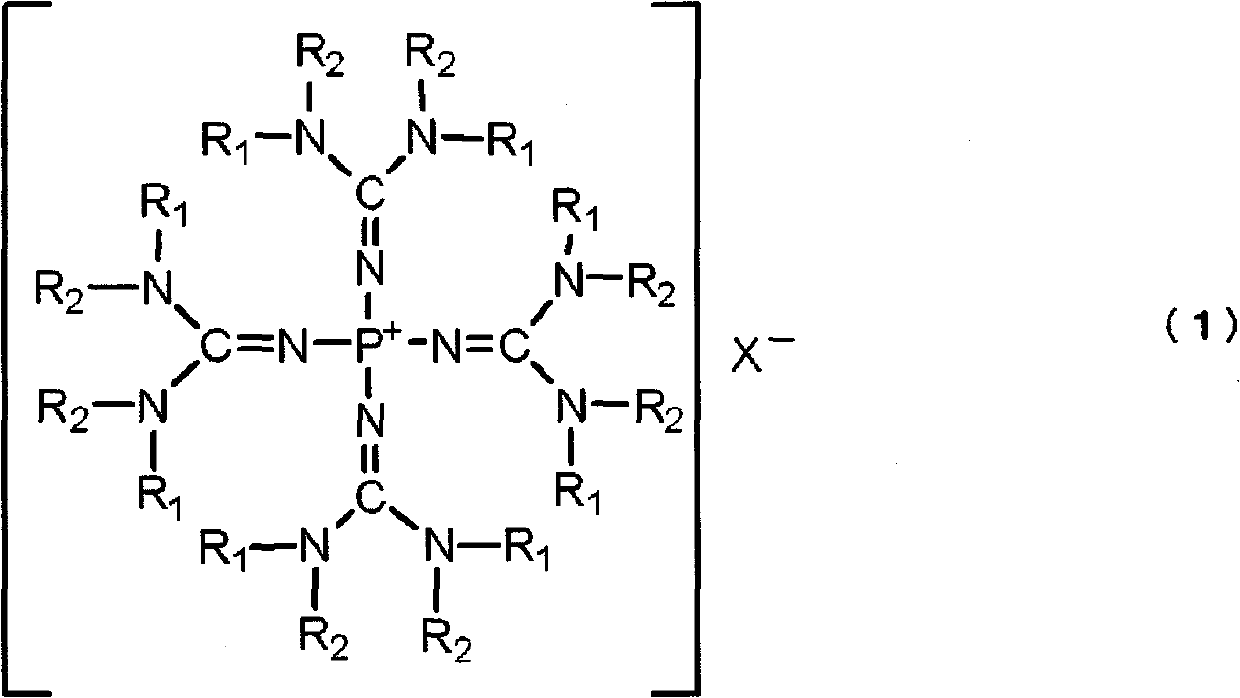
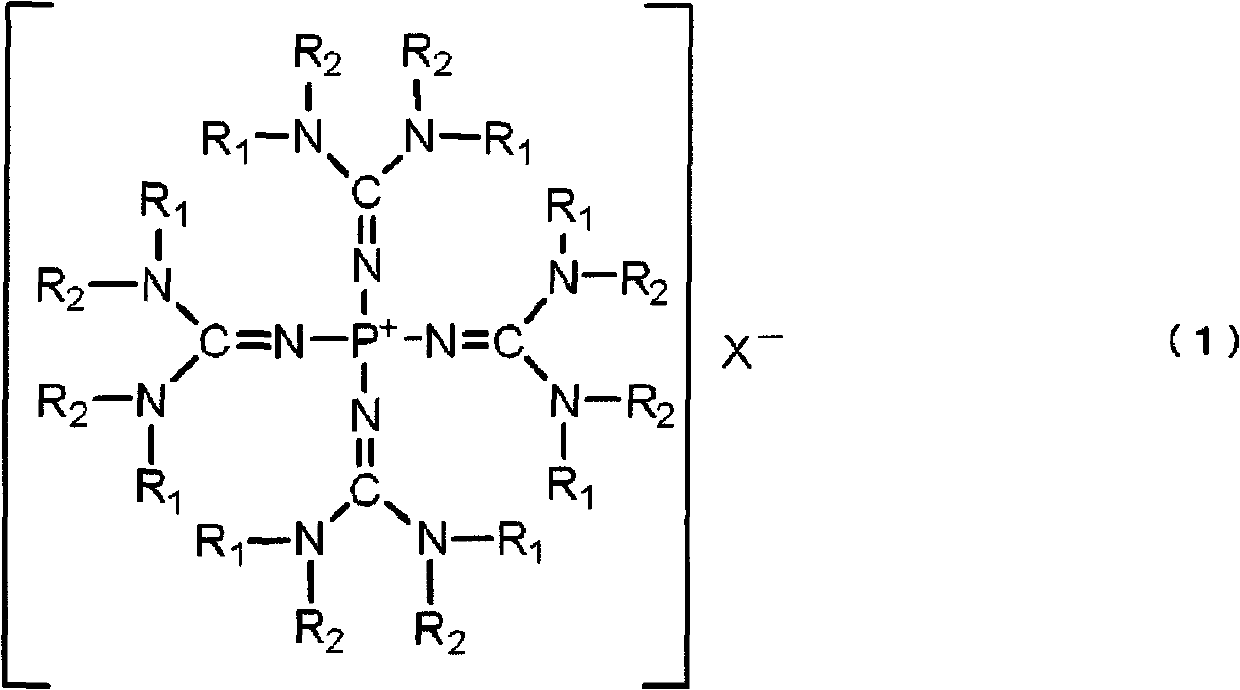
Polyalkylene glycol producing catalyst, and method for producing polyalkylene glycol using same
A technology for polyalkylene glycol and manufacturing method, applied in chemical instruments and methods, organic chemistry, compounds of elements of Group 5/15 of the periodic table, etc. Shows the problems of preparing polyalkylene polyols, etc., to achieve the effect of no residual odor, low total unsaturation, and narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0133] Next, the present invention will be described in more detail by way of examples, but the present invention should not be limitedly interpreted as the following examples. In addition, in the following examples, NMR spectrum, GC-MS, and hydroxyl value were measured as follows.
[0134] Determination of NMR spectrum:
[0135] Measurement was performed using a nuclear magnetic resonance spectrometer (manufactured by JEOL Ltd., trade name: GSX270WB), using tetramethylsilane (TMS) as an internal standard and deuterated chloroform as a deuterated solvent.
[0136] Determination of number average molecular weight:
[0137]Using gel permeation chromatography (GPC) (manufactured by Tosoh Corporation, trade name: HLC 8020GPC), the measurement was performed using tetrahydrofuran as a solvent, and the dissolution curve measured at 40° C. was used as a standard polystyrene-equivalent value.
[0138] GC-MS determination:
[0139] Using a gas chromatography-mass spectrometer (manufa...
Synthetic example 1
[0149] Tetrakis(tetramethylguanidino)phosphonium chloride was synthesized as follows: [(Me 2 N) 2 C=N] 4 P + Cl - (In the formula, Me represents a methyl group. The same applies to the following.).
[0150] Take 4.01g (10.0mmol) of phosphorus pentachloride to a 300ml four-neck flask equipped with a thermometer, dropping funnel, condenser tube and magnetic rotor, and add 60ml of dehydrated toluene (manufactured by Wako Pure Chemicals) to prepare slurry solution. The slurry solution was immersed in a cooling bath cooled to -30°C with dry ice-acetone to lower the internal temperature to -30°C, and then 22.2 g was added dropwise from the dropping funnel over 1 hour under vigorous stirring. (20 mmol) of 1,1,3,3-tetramethylguanidine. After stirring at -30°C for 1 hour, the cooling bath was removed and the temperature was slowly raised to room temperature. Furthermore, this slurry solution was heated at 100 degreeC for 10 hours, and the white slurry solution was obtained. Aft...
Synthetic example 2
[0160] Tetrakis(tetramethylguanidino)phosphonium hydroxide was synthesized as follows: [(Me 2 N) 2 C=N] 4 P + Oh - .
[0161] 3.2 g (6 mmol) of tetrakis[(dimethylamino)imino]phosphonium chloride was dissolved in 100 ml of ion-exchanged water to prepare a 0.06 mol / L solution. This solution was passed through a column (30 mm in diameter, 600 mm in height) filled with 100 ml of hydroxyl-type anion exchange resin (manufactured by Organo Corporation, amberlite IRA410 OH) at room temperature at a flow rate of 300 ml / hour, and then 150 ml of ion exchange resin was passed through at the same flow rate. water. After the effluent was concentrated, it was dried at 40°C and 1mmHg to obtain 3.1g of tetrakis(tetramethylguanidino)phosphonium hydroxide: [(Me 2 N) 2 C=N] 4 P + Oh - of white crystals. The yield is 99%.
[0162] 1 H-NMR measurement result (deuterated solvent: CDCl 3 , internal standard: tetramethylsilane):
[0163] Chemical shift: 2.83 ppm (methyl).
[0164] GC-M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydroxyl value | aaaaa | aaaaa |
| hydroxyl value | aaaaa | aaaaa |
| degree of unsaturation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com