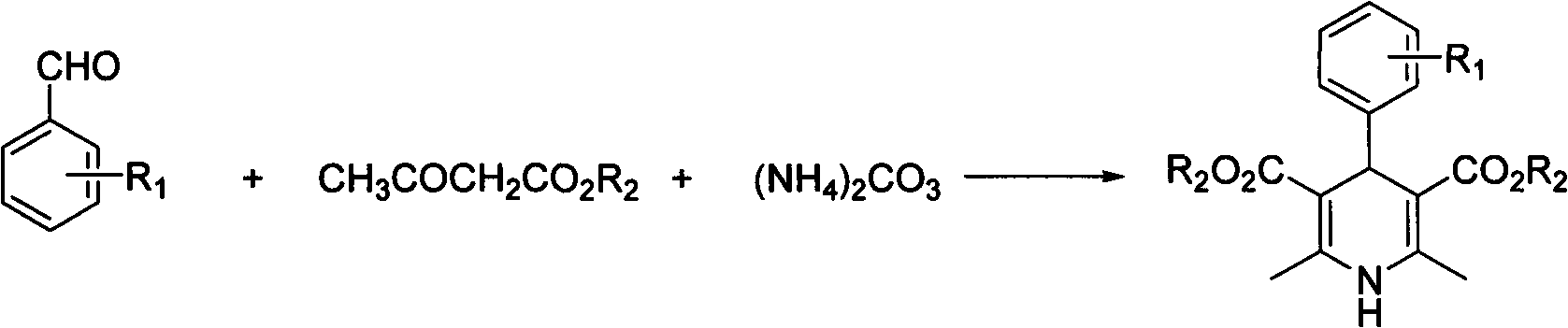Water phase clean synthesis method of 1,4-dihydropyridine compounds
A technology of dihydropyridine and compounds, applied in the field of aqueous phase clean synthesis 1, can solve the problems of easy volatilization of ammonia water, environmental pollution, waste of raw materials, etc., and achieve the effects of easy industrial scale-up, wide source of raw materials, energy saving and emission reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
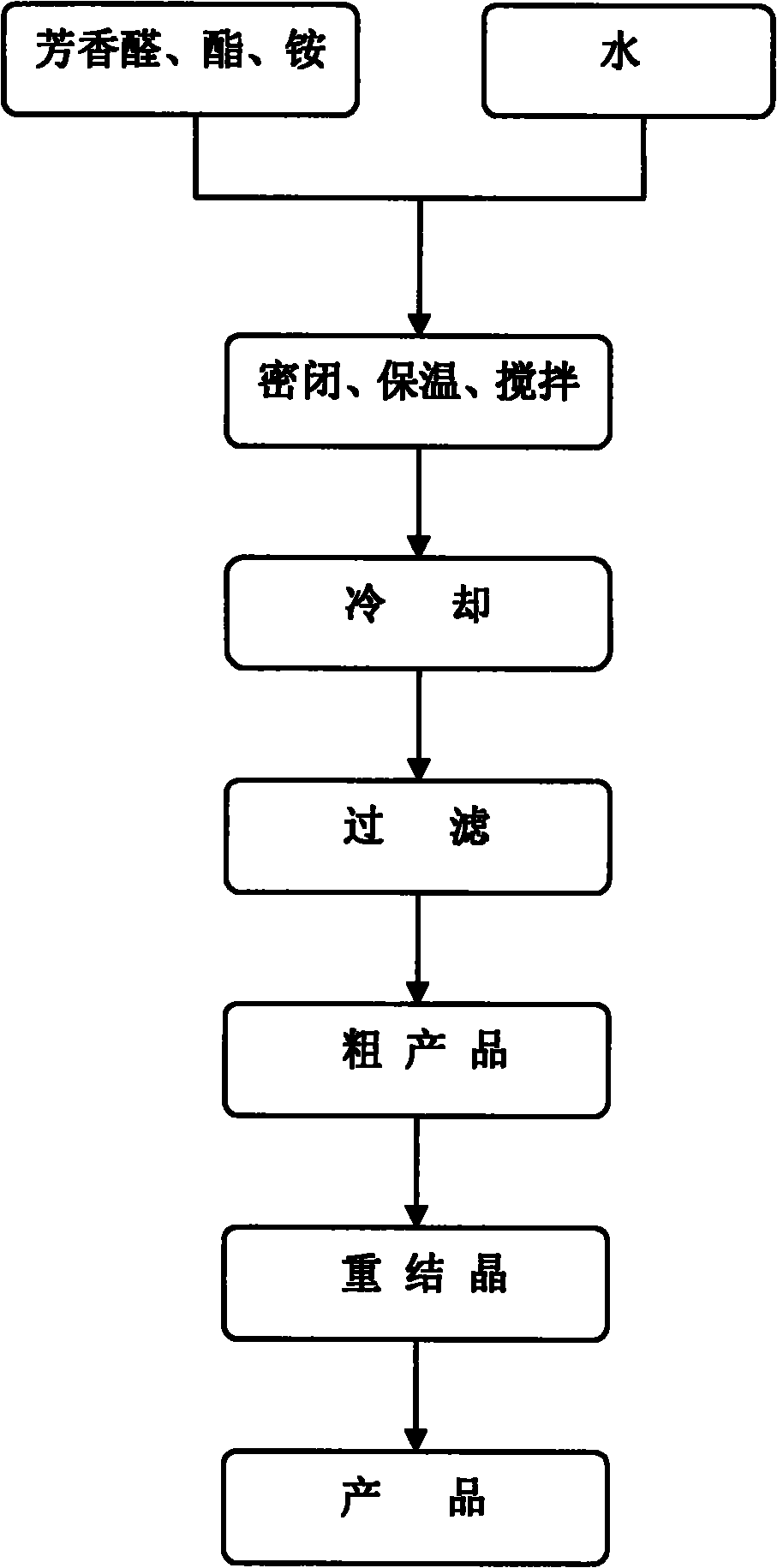
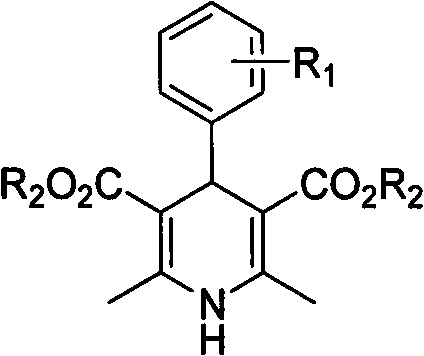
[0020] In a 10mL round bottom flask, add 5mmol (0.53g) of benzaldehyde, 10mmol (1.30g) of ethyl acetoacetate, 5mmol (0.48g) of ammonium carbonate and 5ml of water, and seal the bottle mouth with a glass stopper or rubber stopper to seal the reaction system . Stir at 60°C for 2 hours, cool and filter, and recrystallize from 95% ethanol to obtain 4-phenyl 1,4-dihydropyridine product with a yield of 85%.
Embodiment 2
[0022] In a 25mL round bottom flask, add 5mmol (0.76g) of 2-nitrobenzaldehyde, 10mmol (1.30g) of ethyl acetoacetate, 5mmol (0.48g) of ammonium carbonate and 20ml of water, and stopper the bottle with a glass or rubber stopper Mouth closed reaction system. Stir at 60°C for 2 hours, cool and filter, and recrystallize from 95% ethanol to obtain 4-(2-nitro)phenyl 1,4-dihydropyridine product (nifedipine), with a yield of 86%.
Embodiment 3
[0024] In a 25mL round bottom flask, add 5mmol (0.76g) of 2-nitrobenzaldehyde, 10mmol (1.16g) of methyl acetoacetate, 7.5mmol (0.72g) of ammonium carbonate and 20ml of water, and stopper with a glass or rubber stopper Seal the reaction system at the mouth of the bottle. Mix and stir at 60°C for 2 hours, cool and filter, and recrystallize from 95% ethanol to obtain 4-(2-nitro)phenyl 1,4-dihydropyridine product with a yield of 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com