Azo pigments, coloring compositions, coloring method and colored articles
A technology for coloring compositions and azo pigments, applied in the directions of azo dyes, monoazo dyes, dyeing methods, etc., can solve the problems of insufficient heat resistance, insufficient heat resistance or solvent resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0048] Hereinafter, synthesis examples, examples, and comparative examples are given to further specifically explain the present invention. Among them, "parts" or "%" in the text are quality standards unless otherwise specified.
Synthetic example 1
[0050] (a) Synthesis of coupling component "coupling agent K"
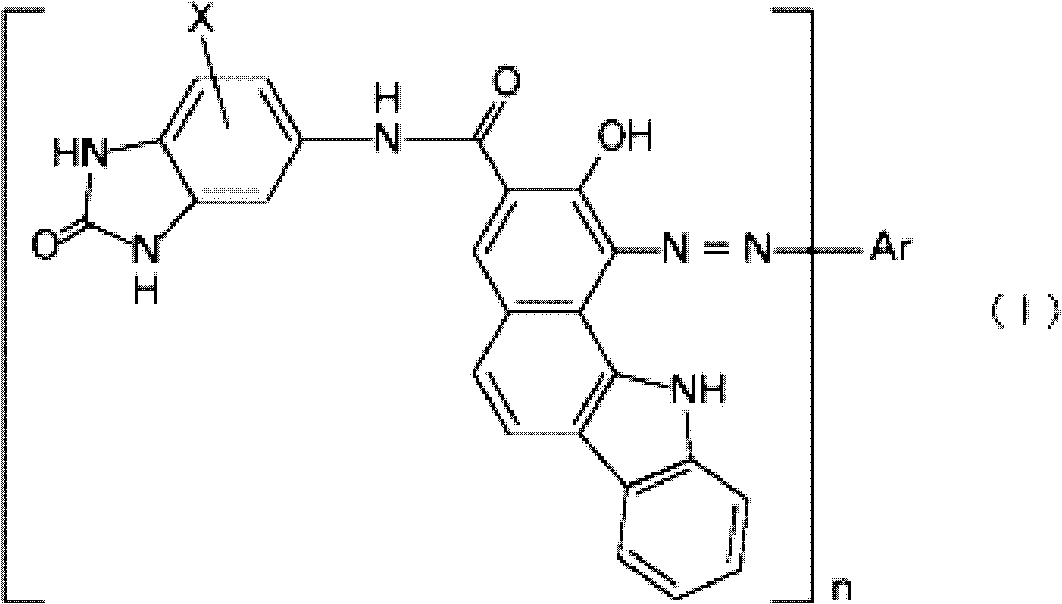
[0051] Add 2,000ml of chlorobenzene, 170.5g (0.57 mol) of sodium 2-hydroxy-11H-benzo[a]carbazole-3-carboxylate and 5-aminobenzimidazolone at 80~85℃ for 1 hour Add 52.4g (0.38mol) of phosphorus trichloride to 56.7g (0.38mol) of the mixture, and then stir at the same temperature for 2 hours, and then at 128-130°C for 25 hours, and then adjust it to alkaline state The solvent was removed by steam distillation, filtered and dried at 50-60°C to obtain the crude 2-hydroxy-11H-benzo[a]carbazole-3-carboxy-N-benzimidazolone-5-amide Things. The crude product was dissolved in 3,000 ml of methanol in which 23 g of sodium hydroxide was dissolved, and after filtering the insoluble components, 68 g of acetic acid was added to precipitate (2 times). The precipitate was filtered, washed with methanol, washed with water, and dried to obtain a coupling agent (hereinafter referred to as "coupling agent K") (melting point: 449°C).
[00...
Synthetic example 2
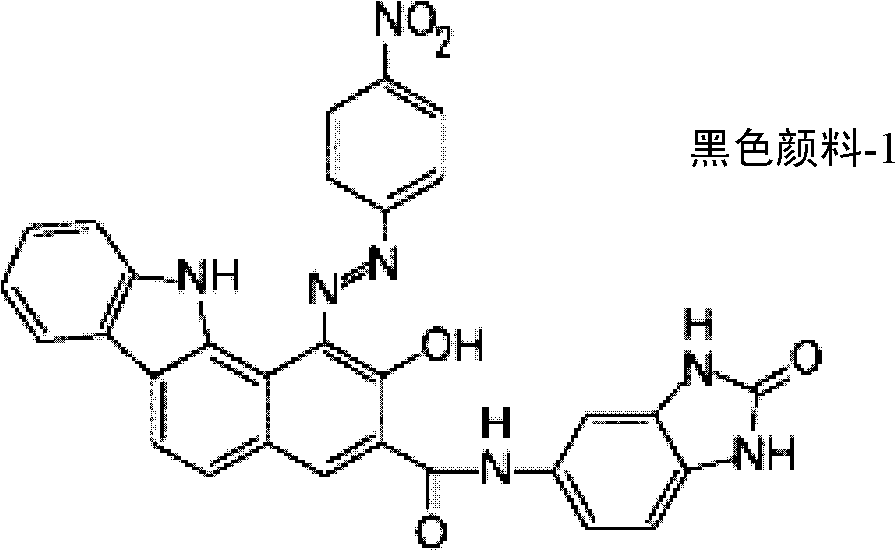
[0060] 2.23 g (0.01 mol) of 1-aminoanthraquinone as an aromatic amine was suspended in 11.3 g of glacial acetic acid, and 3.7 g of concentrated hydrochloric acid was added thereto and stirred. 2.6 g of water was added thereto, maintained at a temperature of 0 to 5°C, 2.0 g of a 40% sodium nitrite aqueous solution was added, and stirred at the same temperature for about 30 minutes to obtain a yellow diazonium salt solution. 4.8 g of sodium acetate trihydrate was added thereto to prepare a diazonium salt solution. Coupling with 4.08 g (0.01 mol) of coupling agent K was carried out in the same manner as in Synthesis Example 1(b) to synthesize a black azo pigment of the following formula. Hereinafter, it is referred to as "black pigment-2".
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com