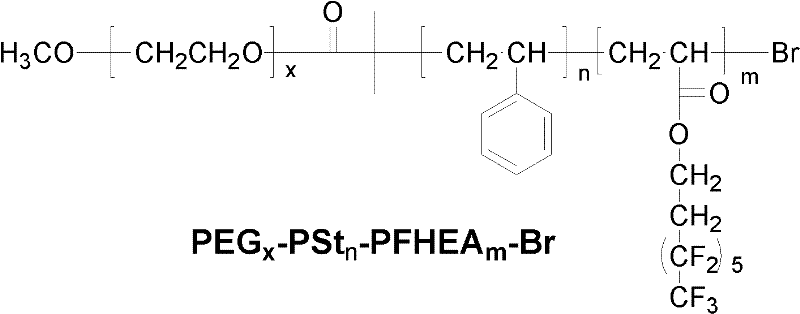
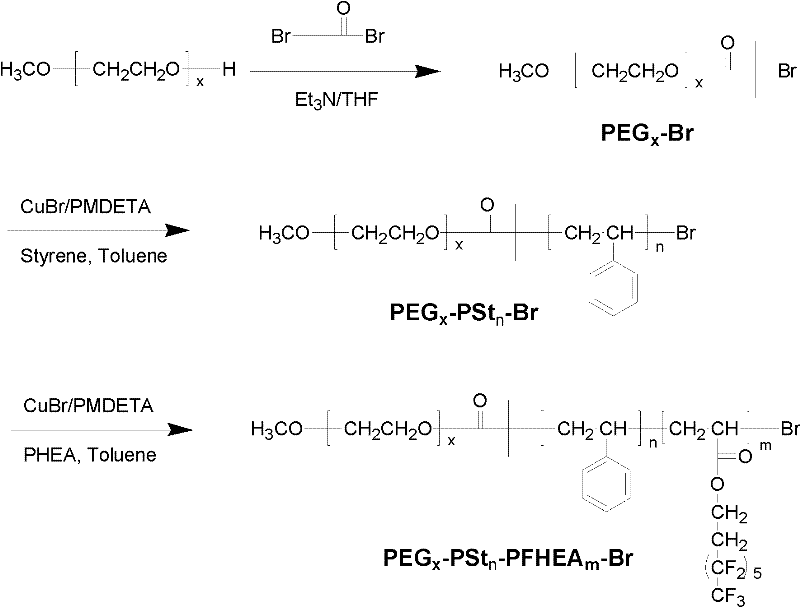
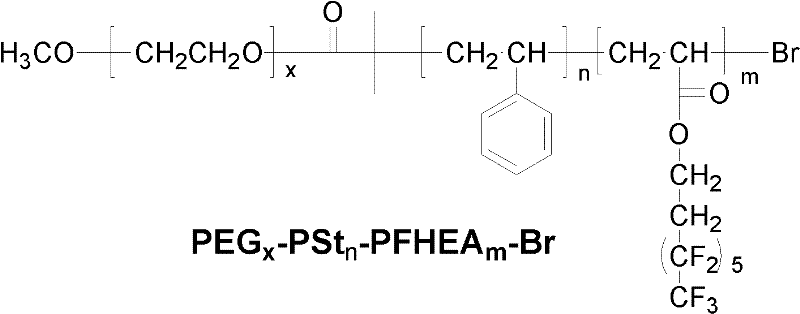
Polyethylene glycol (PEG)-b-polystyrene (PSt)-b-perfluorohexylethyl acrylate (PFHEA) and preparation method thereof
The technology of fluorohexyl ethyl acrylate and polyethylene glycol is applied in the field of amphiphilic triblock copolymer and its synthesis, and can solve the problems of wide molecular weight distribution, no clear molecular structure and composition, low yield and the like, Achieve the effect of narrow molecular weight distribution, low interfacial energy and surface energy, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] 1) Under the protection of nitrogen (purity exceeds 99.99% by mass ratio) atmosphere, 1 part of polyethylene glycol monomethyl ether (number average molecular weight is 750), 1.5 parts of 2-bromoiso Butyryl bromide was reacted with 2 parts of triethylamine under ice-water bath. After the feeding is complete, continue to react at room temperature for 24 hours, filter after the reaction, add dichloromethane to the filtrate, wash with saturated sodium bicarbonate solution, deionized water, hydrochloric acid with a concentration of 0.1mol / L and deionized water successively, and repeat the washing 3 times, liquid separation, the organic layer was dried with anhydrous magnesium sulfate, and then filtered, and the filtrate was distilled off under reduced pressure to remove the dichloromethane solvent to obtain a light yellow active bromine-terminated polyethylene glycol macromolecular initiator;
[0030] 2) Under the protection of nitrogen (purity exceeds 99.99% by mass ratio)...
Embodiment 2
[0034] 1) Except that polyethylene glycol monomethyl ether (the number average molecular weight is 2000) is 1 part, other are the same as step 1 in the embodiment 1);
[0035] 2) Except that the styrene monomer is 300 parts and the reaction temperature is 100° C., the others are the same as step 2 in Example 1);
[0036] 3) Except that the reaction temperature is 80°C and the reaction time is 8h, other steps 3) are the same as in Example 1;
[0037] A fluorine-containing tri-block copolymer of polyethylene glycol-b-polystyrene-b-polyperfluorohexyl ethyl acrylate was obtained.
Embodiment 3
[0039] 1) Except that 2-bromoisobutyryl bromide is 3 parts and triethylamine is 4.5 parts, other are the same as step 1 in Example 1);
[0040] 2) except that styrene monomer is 300 parts, reaction temperature 90 ℃, other are the same as step 2 in embodiment 1);
[0041] 3) Except that the reaction temperature is 80°C and the reaction time is 8h, other steps 3) are the same as in Example 1;
[0042] A fluorine-containing tri-block copolymer of polyethylene glycol-b-polystyrene-b-polyperfluorohexyl ethyl acrylate was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com