Completely degradable polymer medicine elution stent and preparation method thereof
A technology for degrading polymers and eluting stents, which is applied in the field of preparation of fully degradable polymer drug-eluting stents, can solve problems such as insufficient mechanical support performance, uneven stent structure, unfavorable polymer flow, etc., and achieve good bioavailability Degradation, absorption, energy absorption, good biocompatibility, and the effect of inhibiting intravascular restenosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
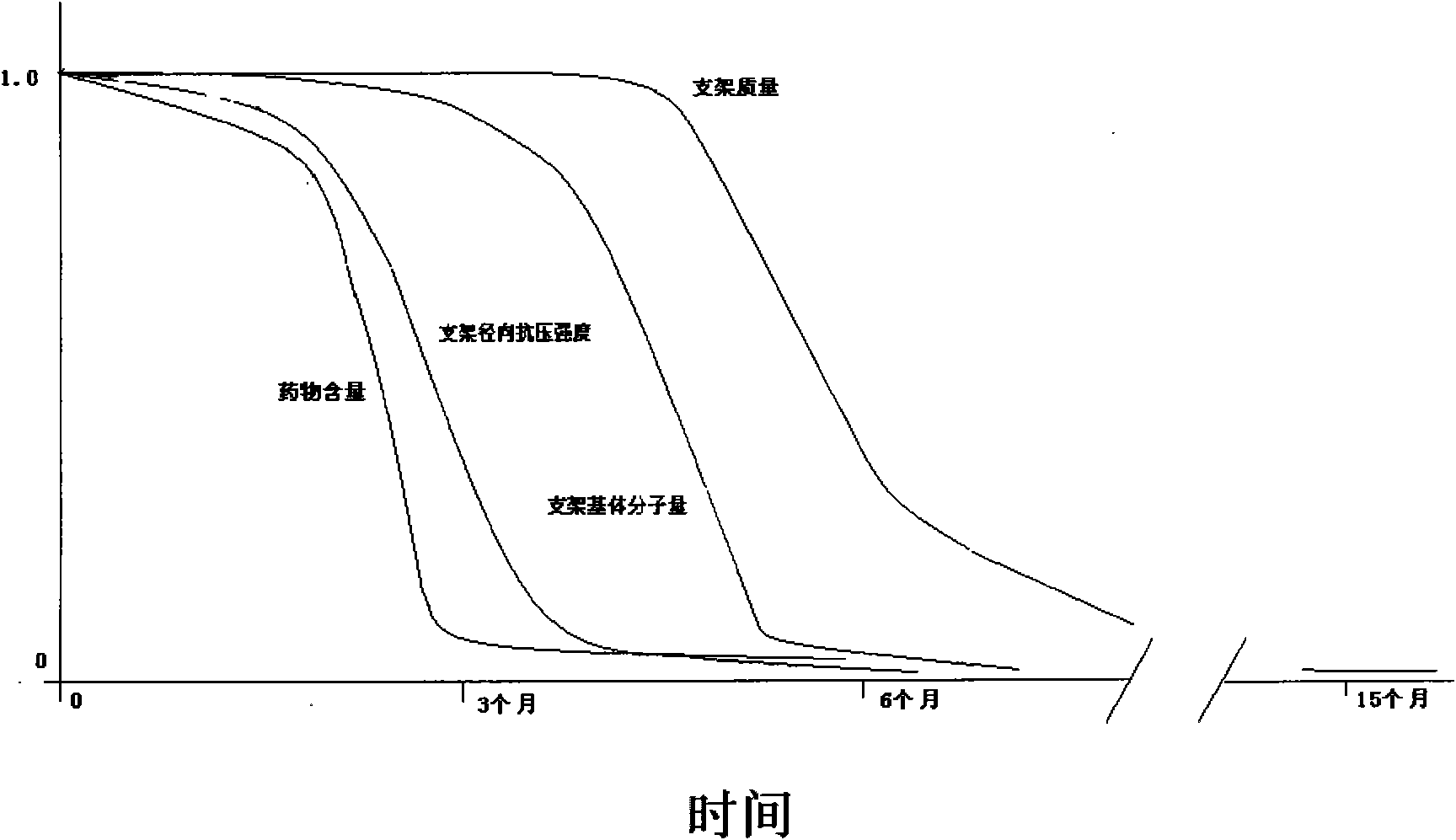
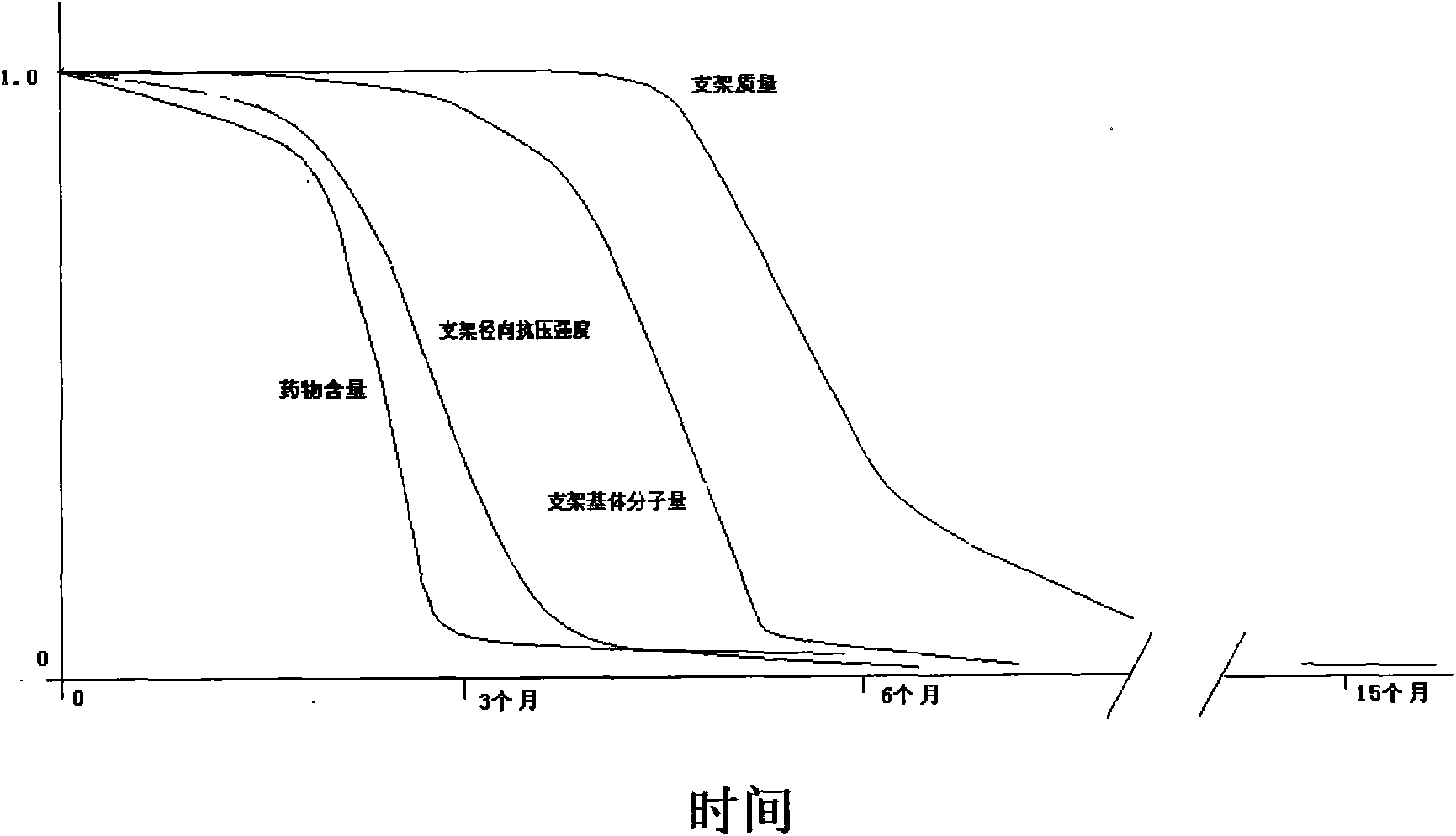
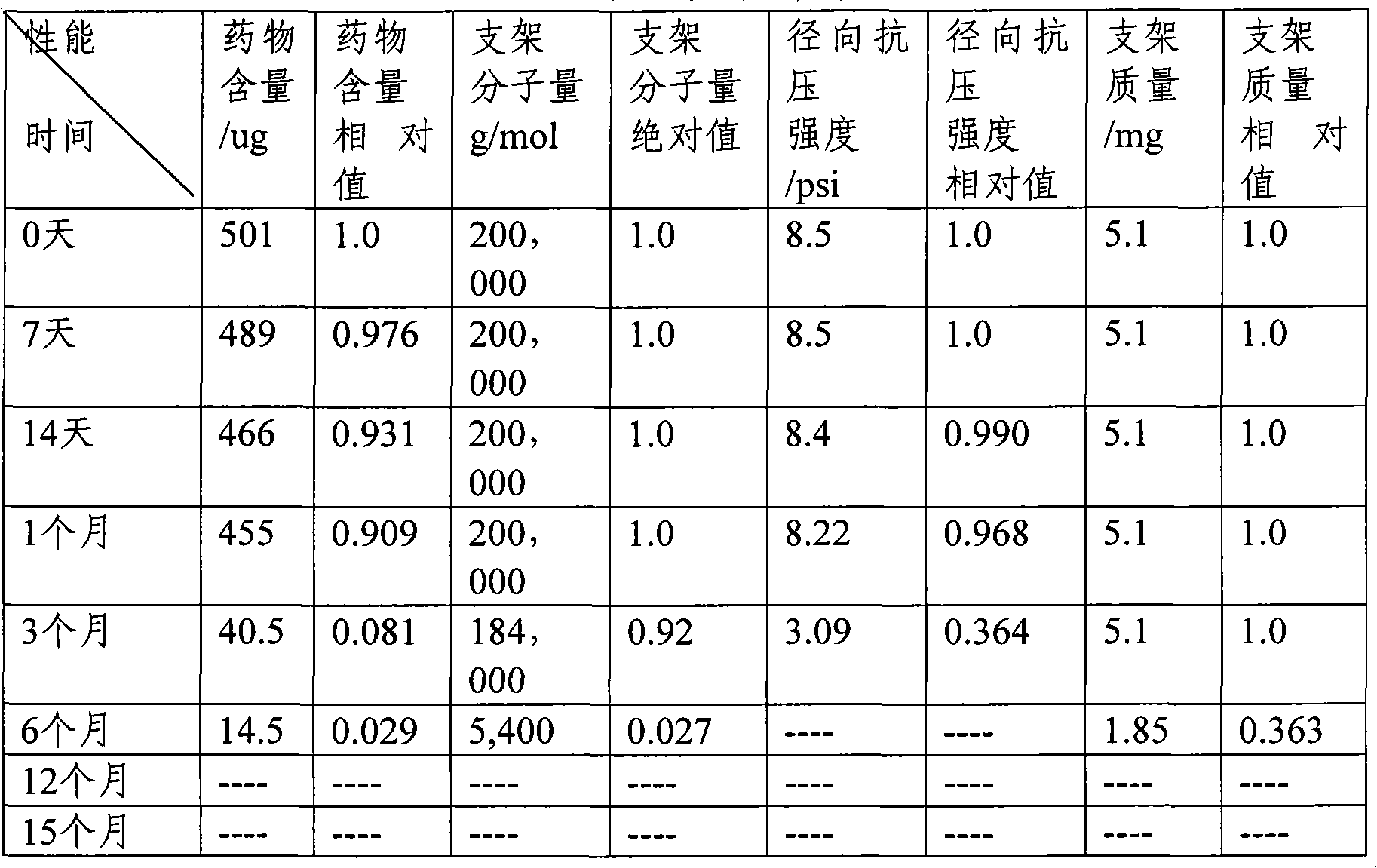
Image
Examples
Embodiment 1
[0049] (1) Select L-polylactic acid PLLA with an average molar molecular weight of 200,000 as the scaffold matrix material, heat the material to 200°C in a plastic molding extruder (the melting point of PLLA is 180°C), and wait until the material is completely melted to a uniform state , fill it into the pipe forming mold, the outer diameter of the injection mold is 5mm, the wall thickness is 0.3mm, and slowly cool at a cooling rate of 1°C / min until the internal temperature of the material drops to room temperature, open the mold to remove the pipe from the mold Take it out from the middle to obtain a pipe with an outer diameter of 5 mm and a wall thickness of 0.3 mm. Because the cooling rate is slow enough, the obtained PLLA pipe has high crystallinity and high strength. At the same time, in order to improve the demoulding efficiency of the pipe, a high-temperature silicone ester mold release agent is used.
[0050] (2) Use a laser engraving instrument with a XeCl gas excimer...
Embodiment 2
[0055] (1) The polylactic acid-glycolic acid copolymer PLGA (wherein PLA:PGA is 90%:10%) with an average molar molecular weight of 500,000 is selected as the scaffold matrix material, and the material is heated to 200° C. in a plastic extruder (PLGA The melting point temperature is 180°C). After the material is completely melted to a uniform state, it is extruded from the molding die. The size of the extrusion molding die is 3mm in outer diameter and 0.15mm in wall thickness, and slowly cooled at a cooling rate of 5°C / min, using segmental cooling, the cooling medium is air cooling. That is, a pipe with an outer diameter of 3 mm and a wall thickness of 0.15 mm is obtained.
[0056] (2) Use a laser engraving instrument with a KrF gas excimer laser light source with a wavelength of 248nm to laser engrave a fully degradable polymer tube into a stent, control the power within the range of 0.35W to 0.4W, and have a pulse width of 23ns to obtain the stent matrix .
[0057] (3) Usi...
Embodiment 3
[0061] (1) Select polyglycolic acid PGA with an average molar molecular weight of 300,000 as the scaffold matrix material, and heat the material to 240°C (the melting point of PGA is 230°C) in a plastic molding injection molding machine. After the material is completely melted to a uniform state, Fill it into the pipe forming mold, the outer diameter of the injection mold is 3mm, the wall thickness is 0.2mm, and slowly cool at a cooling rate of 10°C / min until the internal temperature of the material drops to room temperature, open the mold to remove the pipe from the mold Take it out to obtain a pipe with an outer diameter of 3 mm and a wall thickness of 0.2 mm. At the same time, in order to improve the demoulding efficiency of the pipe, a high-temperature silicon ester mold release agent is used.
[0062] (2) Use a laser engraving instrument with a XeCl gas excimer laser source with a wavelength of 308nm to laser engrave a fully degradable polymer tube into a stent. The power ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com