Formula, preparation method and use of azithromycin liposome combined medicament
A technology of azithromycin lipid and liposome, which is applied in the direction of pharmaceutical formulation, liposome delivery, and medical preparations of non-active ingredients, etc. It can solve the problems of increasing leakage rate, batch fluctuation, energy consumption and time-consuming, etc., and improve the treatment Index, the realization of zero discharge of three wastes, and the effect of saving workshops
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
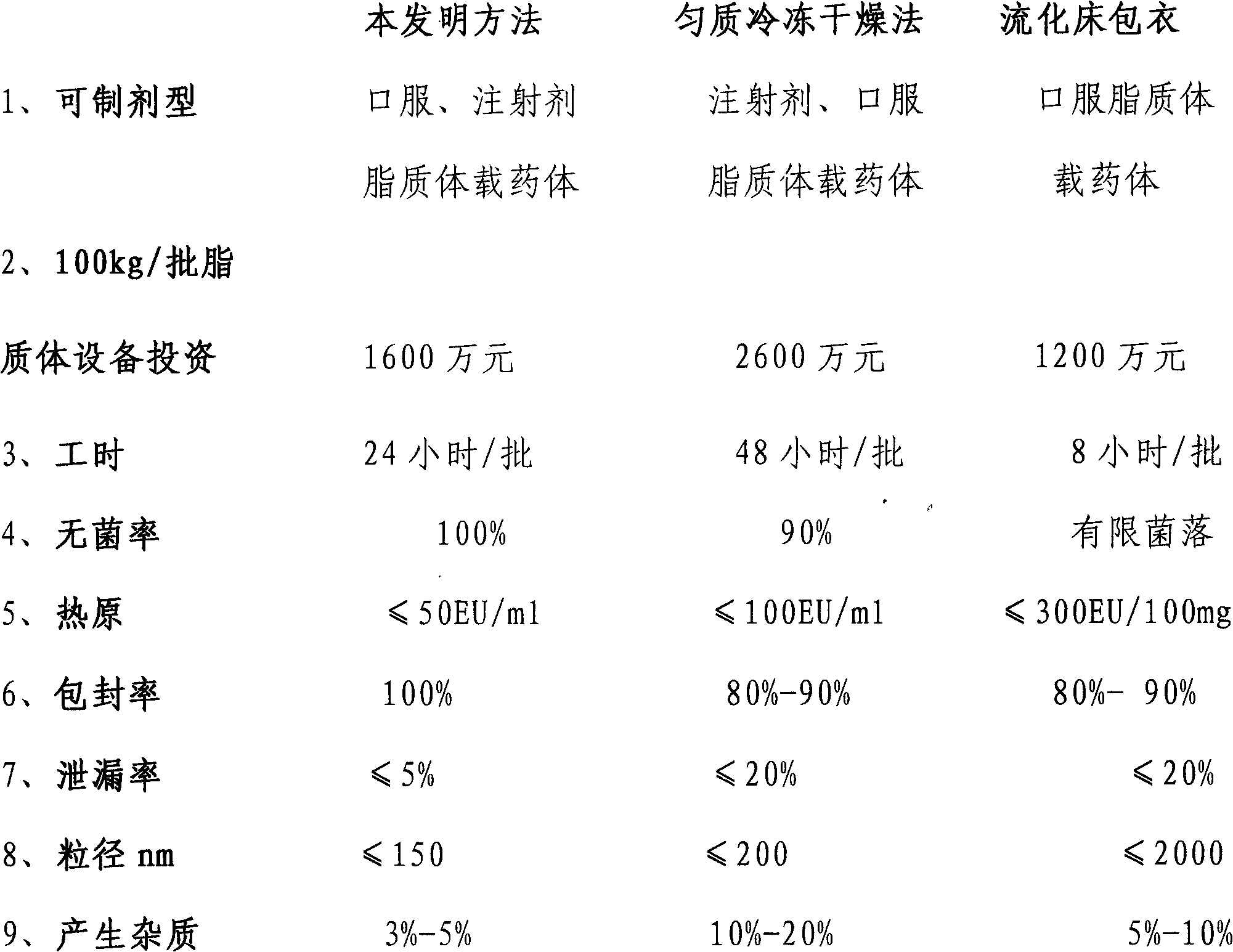
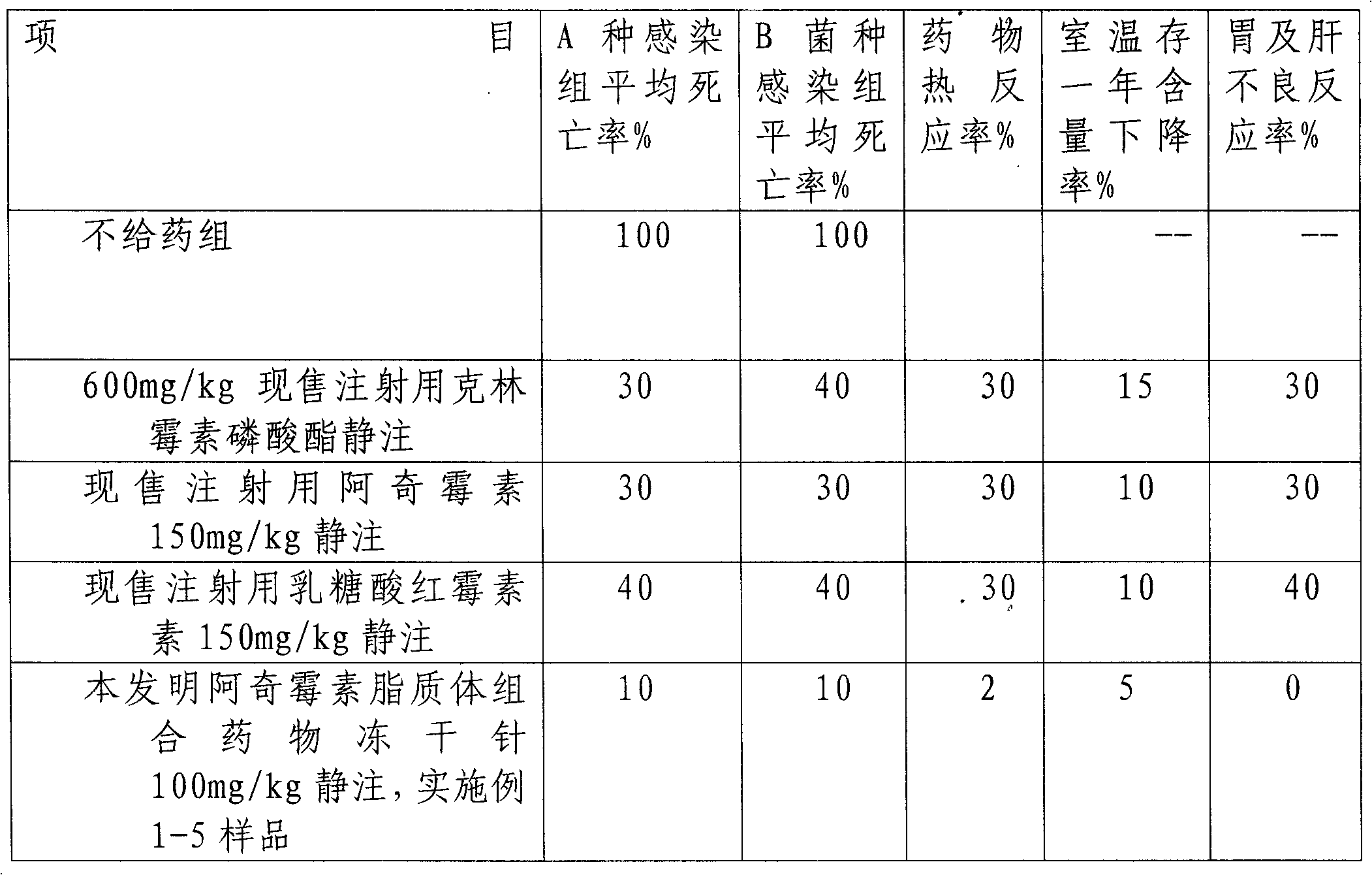
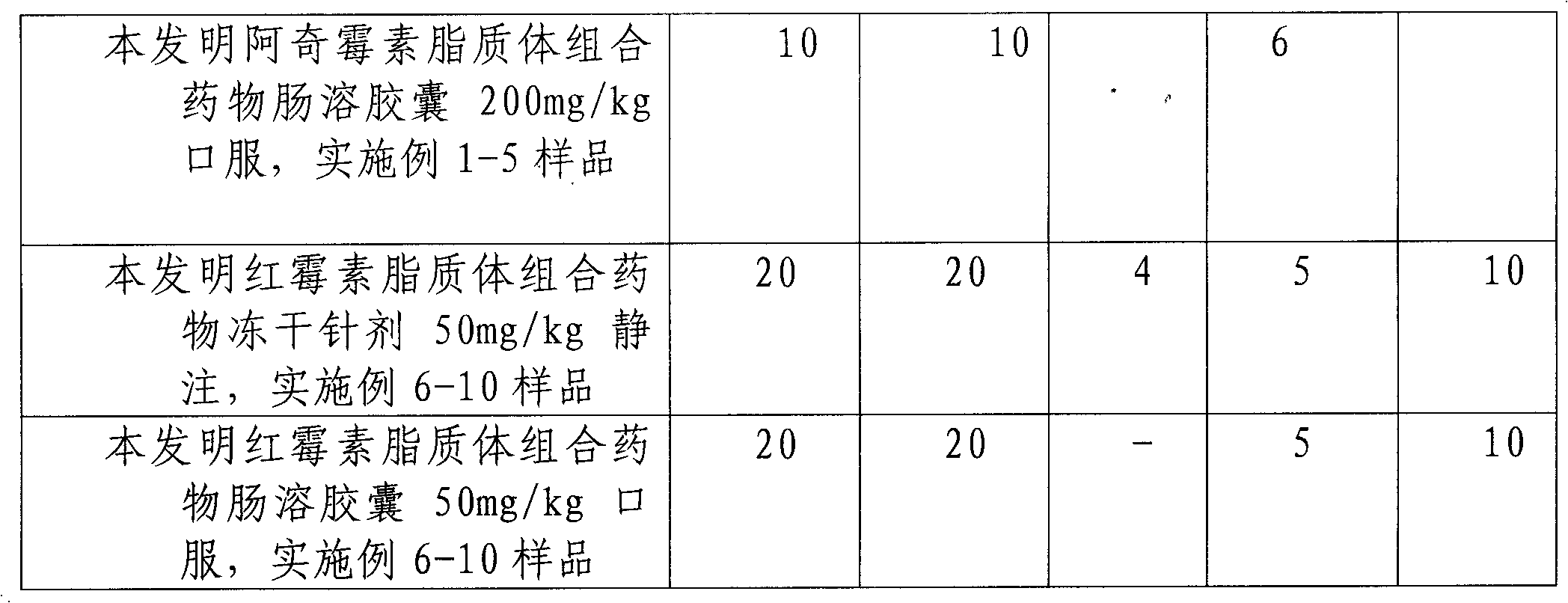
Image
Examples
Embodiment 1
[0057] The raw material drug is a liposome combination drug with strong fat solubility. The molar ratio of each medicinal raw material is as follows:
[0058] 1. The raw material drug is azithromycin 0.05
[0059] 2. Phospholipid raw materials are soybean lecithin and polyene phosphatidylcholine
[0060] Mole ratio 1:0.5 composition 0.50
[0061] 3. The antioxidant is reduced glutathione 0.01
[0062] 4. The molecular diluent of phospholipid membrane is dimercaprol 1.00
[0063] 5. The dispersant and excipient of liposome drug-carrying body is xylitol 2.00
[0064] 6. Surfactant is sodium dehydrocholate 0.01
[0065] 7. Ethanol 80% (v / v) (when dry, evaporate to the fullest) appropriate amount
[0066] 8. Phosphate buffer solution for injection 0.05M pH value 5.0-8.0 appropriate amount
[0067] 9. Equal volumes of water for injection (when dry, evaporate to the limit) and phosphate buffer for injection
[0068] The standard preparation steps and methods of this embodimen...
Embodiment 2
[0078] The raw material drug is a liposome combination drug with strong fat solubility. The molar ratio of each medicinal raw material is as follows:
[0079] 1. The API is Azithromycin 0.20
[0080] 2. Phospholipid raw materials are soybean lecithin and polyene phosphatidylcholine
[0081] 5:0.5 molar composition 4.00
[0082] 3. The antioxidant is reduced glutathione 0.06
[0083] 4. The molecular diluent of phospholipid membrane is dimercaprol 6.00
[0084] 5. The dispersant and excipient of liposome drug-carrying body is xylitol 8.00
[0085] 6. Surfactant is sodium dehydrocholate 0.05
[0086] 7. Ethanol 80% (v / v) (when dry, evaporate to the fullest) appropriate amount
[0087] 8. Phosphate buffer solution for injection 0.05M pH value 5.0-8.0 appropriate amount
[0088] 9. Equal volumes of water for injection (when dry, evaporate to the limit) and phosphate buffer for injection
[0089] The standard preparation steps and method of this embodiment are the same as in...
Embodiment 3
[0091] The raw material drug is a liposome combination drug with strong fat solubility. The molar ratio of each medicinal raw material is as follows:
[0092] 1. The raw material drug is azithromycin 0.05
[0093] 2. Phospholipid raw materials are soybean lecithin and polyene phosphatidylcholine
[0094] Mole ratio 2:0.5 composition 4.00
[0095] 3. The antioxidant is reduced glutathione 0.01
[0096] 4. The molecular diluent of phospholipid membrane is dimercaprol 6.00
[0097] 5. The dispersant and excipient of liposome drug-carrying body is xylitol 2.00
[0098] 6. Surfactant is sodium dehydrocholate 0.05
[0099] 7. Ethanol 80% (v / v) (when dry, evaporate to the fullest) appropriate amount
[0100] 8. Phosphate buffer solution for injection 0.05M pH value 5.0-8.0 appropriate amount
[0101] 9. Equal volumes of water for injection (when dry, evaporate to the limit) and phosphate buffer for injection
[0102] The standard preparation steps and method of this embodiment...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com