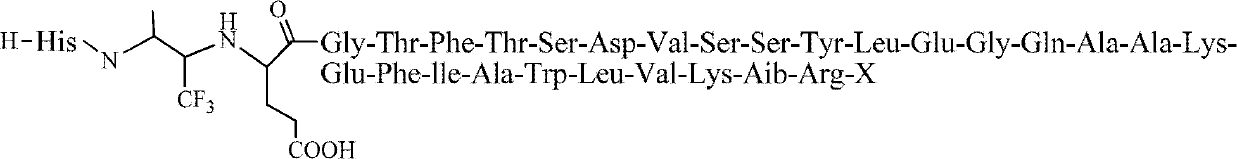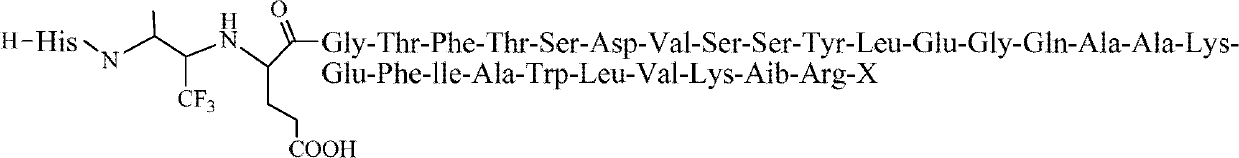GLP-1 (glucagon-like peptide-1) derivative and application thereof
A drug and compound technology, applied in the field of glucagon-like peptide-1 derivatives, can solve the problems of limiting the clinical application of GLP-1, instability of GLP-1, etc., and achieve remarkable effects, long plasma half-life, and chemical properties stable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 prepares the compound shown in structural formula I
[0028] I, synthetic intermediate (called Dimer)
[0029] The structure of Dimer is as follows:
[0030]
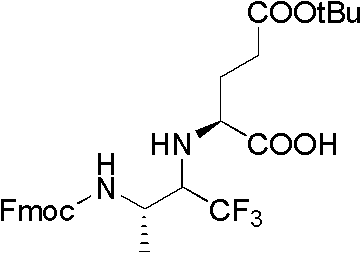
[0031] The specific synthesis method is as follows:
[0032] 1. Preparation of compound 01·HCl
[0033] Below -10°C, add 80ml of thionyl chloride dropwise to 250ml of methanol, complete the dropwise addition within 2 hours, and stir at room temperature for 1 hour. Add 40 g of L-alanine and stir overnight. Then, the temperature was raised to reflux for 4 hours. After cooling, the solvent was removed under reduced pressure to constant weight to obtain 80 g of crude compound 01·HCl.
[0034] 2. Preparation of Compound 02
[0035] 40g of compound 01·HCl was dissolved in 350ml of DMF, 113g of benzyl bromide was added, and 150g of anhydrous potassium carbonate was added under stirring, and after stirring for 2 hours, the reaction was kept at 50°C for 2 hours. Then, the reaction solution was extra...
Embodiment 2
[0065] Example 2 Determination of glucose tolerance
[0066] 1. Test group:
[0067] 90 ICR (Institute of Cancer Research) mice, all male, were divided into three batches according to body weight, 30 in each batch. Each batch of mice was fasted overnight and divided into two groups according to blood sugar: Vehicle group and compound group represented by structural formula I (compound group for short). The Vehicle group was only injected with normal saline, and the compound group was injected with the compound represented by structural formula I in the normal saline.
[0068] 2. Test process:
[0069] The first batch of animals: mice were grouped according to blood sugar after fasting overnight, administered subcutaneously on Day 1, the dose of the compound group was 0.3 mg / kg, and mice in each group were given 2 g / kg sugar load 2 hours after administration After 30 and 60 minutes, blood was collected to measure blood sugar. On Day 4, mice in each group were fasted overnig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com