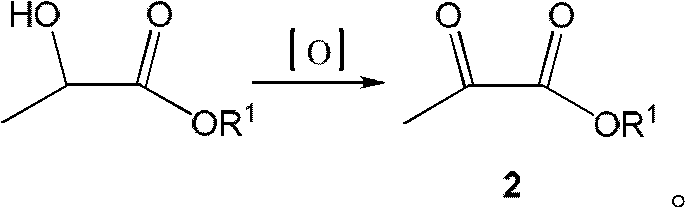
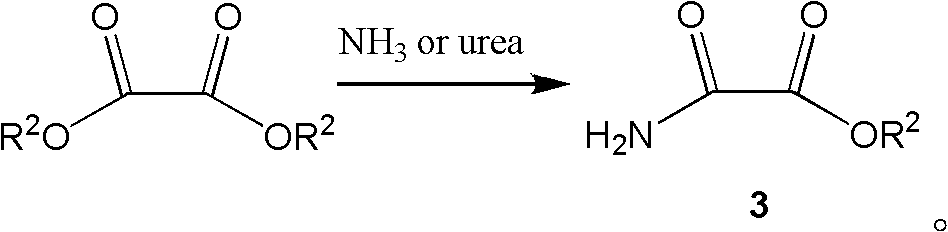
Preparation method of N-alkyloxy oxalyl alanine alkyl ester
A technology of alkoxy oxalylalanine alkyl ester and oxalyl alanate, which is applied to the preparation of carboxylic acid amides, the preparation of organic compounds, chemical instruments and methods, etc., which can solve the problem of energy consumption and unsuitability for industrialization Production, expensive and other issues, to achieve the effect of low total cost, large-scale industrial production and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Under nitrogen protection, add 5.81g (50mmol) of ethyl pyruvate, 5.85g (50mmol) of ethyl oxamate, 100mL of 1,2-dichloroethane and 1g (16.65mmol) of acetic acid to the reaction flask at room temperature, and stir at room temperature overnight.
[0033] The next day, 14 g of sodium triacetoxyborohydride (66.06 mmol) was added to the reaction solution, and stirring was continued for 3 hours.
[0034] Add dilute sodium carbonate solution under ice bath to adjust pH=7-8, let stand to separate layers, wash the organic layer with water 30mL*3; concentrate the organic layer to obtain 10.7g of light yellow slurry; pump vacuum distillation, distill off 150 The fraction below °C yielded 10.43 g (48.02 mmol) of the target product with a GC purity of 99.13% and a yield of 96.03%.
Embodiment 2
[0036] Under nitrogen protection, add 5.81g (50mmol) of ethyl pyruvate (50mmol), 5.85g (50mmol) of ethyl oxamate, 0.5g of p-toluenesulfonic acid (2.90mmol) and 100mL absolute ethanol to the reaction flask at room temperature, and stir at room temperature overnight.
[0037] The next day, 0.1 g of palladium carbon was added, and hydrogen gas was passed through the reaction solution at room temperature for 3 hours.
[0038] Filter the reaction solution, concentrate to dry ethanol, add 100mL of petroleum ether, separate layers, wash the organic layer with 30mL*3 of water; concentrate the organic layer to obtain 10.9g of light yellow syrup; 9.11 g (41.94 mmol) of the target product was obtained, the GC purity was 99.35%, and the yield was 83.88%.
Embodiment 3
[0040] Under nitrogen protection, 5.81 g (50 mmol) of ethyl pyruvate, 5.85 g (50 mmol) of ethyl oxalate, 0.5 g of p-toluenesulfonic acid (2.90 mmol), and 0.05 g of 10% palladium carbon were added to the autoclave at room temperature and 100 mL of absolute ethanol, and stirred overnight at room temperature.
[0041] The nitrogen gas was removed, the hydrogen gas was passed through, and the pressure was maintained at 1Mpa, and the reaction was stirred for 2 hours.
[0042] Replace with nitrogen, filter the reaction solution, then concentrate dry ethanol, add 100mL petroleum ether, separate layers, wash the organic layer with water 30mL*3; concentrate the organic layer to obtain 10.7g of light yellow slurry; The fraction below °C yielded 9.98 g (45.94 mmol) of the target product with a GC purity of 99.16% and a yield of 91.89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com