Chlorophyllin salt compound and preparation method thereof
A chlorophyll compound, compound technology, applied in the directions of organic chemistry, pharmaceutical combination, pharmaceutical formulation, etc., can solve the problems of incomplete clarity of active ingredients, poor water solubility of porphyrin salts, and ineffectiveness, etc., and achieve beneficial effects on the human body. The effect of health, strong water solubility, and low dark toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
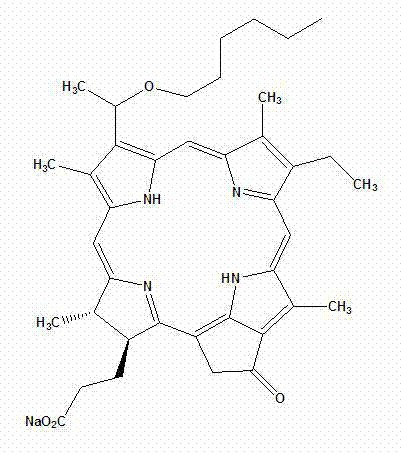
[0037] A chlorophyllin compound, the general formula is as follows:
[0038]
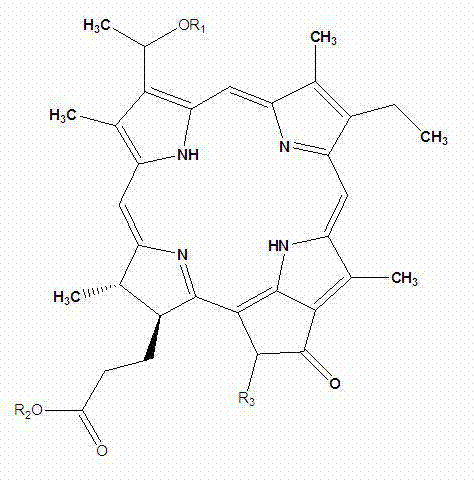
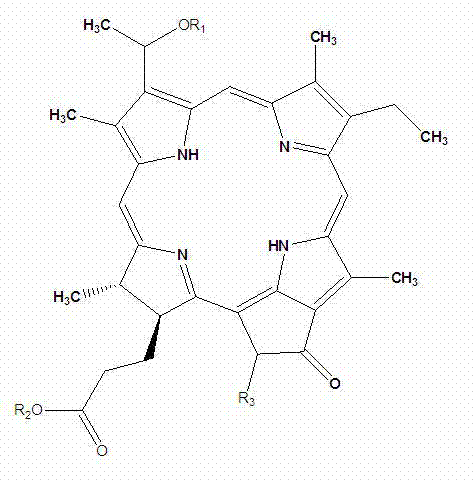
[0039] Among them, R 1 independently selected from C 1-18 Alkyl, C 1-18 Alkenyl, C 1-8 Alkoxy and C 1-18 Acyl, which is optionally substituted by one or more substituents selected from halogen and hydroxy; R 2 for Na + , sodium carboxylate, or glucoside; R 3 Is H, carboxyl, or sodium carboxylate.
[0040] In a preferred embodiment, R 1 independently selected from CH 2 (CH 2 ) 4 CH 3 , R 2 for Na + , sodium carboxylate, or glucoside, R 3 is H or carboxyl.
[0041] [0023] Examples of water-soluble chlorophyllin compounds in this embodiment include but are not limited to:
[0042]
[0043] (I)
[0044]
[0045] (II)
[0046] The preparation method of the above-mentioned chlorophyllin salt compound comprises the following steps:
[0047] 1. Convert pheophorbide into acidic porphyrin;
[0048] II. Derivatizing the acidic porphyrin to obtain a complex derivative acidic porphy...
Embodiment 2
[0062] In this example, 2-desvinyl-2-(1-hexylhydroxyethyl)-pyropheophorbide-a sodium (1) was prepared from silkworm excrement chlorophyll.
[0063] Ⅰ. Convert pheophorbide into acidic porphyrin:
[0064] A, preparation of pheophorbide-a methyl ester:
[0065] Dissolve 200g silkworm excrement chlorophyll in 4000mL methanol solution containing 5% sulfuric acid; then stir under nitrogen protection at 15-25°C in the dark for 12 hours; then filter; add water and dichloromethane for extraction; The organic phase is washed with water; then dried and concentrated under reduced pressure; and then the obtained concentrate is subjected to silica gel column chromatography separation treatment. In the chromatography separation treatment, the eluent is petroleum ether and ethyl acetate ester, the volume ratio of the petroleum ether and ethyl acetate was 3:1, and the dark green solid A6.9g was obtained, which contained the pheophorbide-a methyl ester, and the yield was 85%. UV-vis (CHCl 3...
Embodiment 3
[0077] In this example, 2-desvinyl-2-(1-hexylhydroxyethyl)-pheophorbide-a disodium (2) was prepared from silkworm excrement chlorophyll.
[0078] Ⅰ. Convert pheophorbide into acidic porphyrin:
[0079] A. Preparation of pheophorbide-a methyl ester:
[0080] Dissolve 100g silkworm excrement chlorophyll in 2000mL methanol solution containing 5% sulfuric acid; then stir under nitrogen protection at 15-25°C and avoid light for 12 hours; then filter; add water and dichloromethane for extraction; The organic phase is washed with water; then dried and concentrated under reduced pressure; and then the obtained concentrate is subjected to silica gel column chromatography separation treatment. In the chromatography separation treatment, the eluent is petroleum ether and ethyl acetate , the volume ratio of petroleum ether and ethyl acetate was 3:1, and 3.50 g of dark green solid A was obtained, which contained the pheophorbide-a methyl ester, and the yield was 85%. UV-vis (CHCl 3 ) λm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com