Method for catalyzing benzene and hydrogen peroxide to synthesize phenol by using Cu-loading Schiff base
A technology for catalyzing benzene and hydrogen peroxide with a Schiff base, which is applied in chemical instruments and methods, chemical/physical processes, physical/chemical process catalysts, etc., can solve the problems of low catalyst synthesis yield and selectivity, and achieve easy operation , high phenol yield, and the effect of increasing phenol yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Based on 1g of MCM-41, the catalyst Cu(salen) / MCM-41 was synthesized according to the following steps. 1g of MCM-41 molecular sieve was suspended in 15mL of anhydrous toluene, 5mL of 3-aminopropyltriethoxysilane was added, and nitrogen protection , stirred and refluxed for 48h, filtered, washed with toluene and diethyl ether in turn, and dried in vacuo to obtain 1.3g of MCM-41-NH 2 Molecular sieve. 0.45g Cu(salen) dissolved in 60mL CHCl 3 In, add 1g MCM-41-NH 2 , the suspension was stirred at room temperature for 24 h, filtered, dried, extracted with methanol until the solution no longer changed color, and dried in vacuum to obtain Cu(salen) / MCM-41.
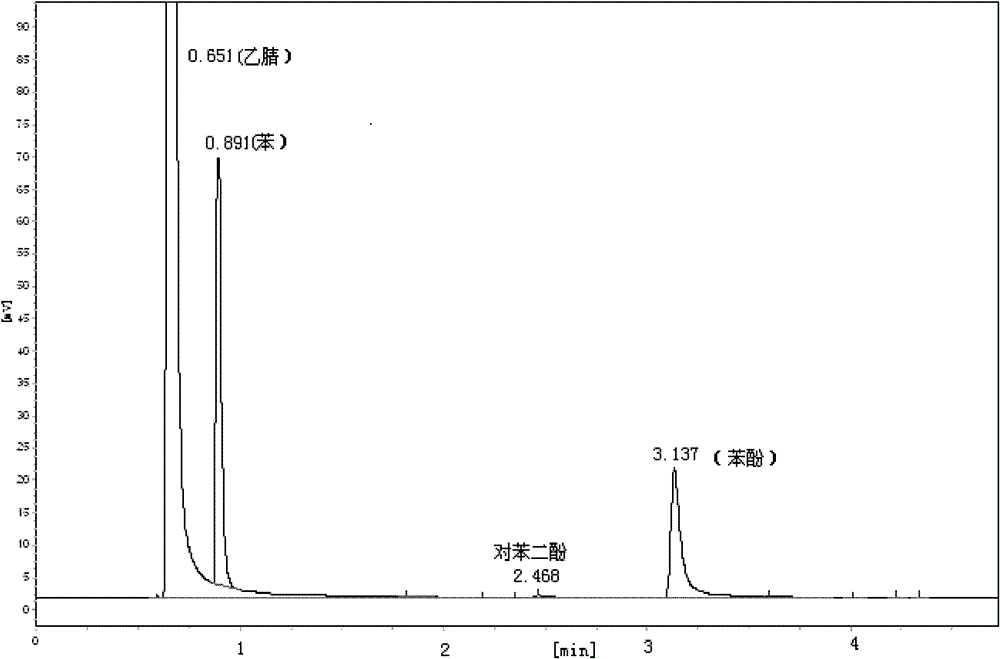
[0022] In the there-necked flask, the mass ratio of 2.16% Cu(salen) / MCM-41, 0.51% triphenylphosphine, 8.65% benzene, 76.2% acetonitrile, 13.0% 30% H 2 o 2 , heated to 65°C, and refluxed for 6h under magnetic stirring. After the reaction was completed, it was cooled to room temperature, the catalyst was filtered, and t...
example 2
[0024] Based on 1g of MCM-41, the catalyst Cu(saloph) / MCM-41 was synthesized according to the following steps. 1g of MCM-41 molecular sieve was suspended in 15mL of anhydrous toluene, 5mL of 3-aminopropyltriethoxysilane was added, and under nitrogen protection , stirred and refluxed for 10h, filtered, washed with toluene and diethyl ether in turn, and dried in vacuum to obtain 1.3g MCM-41-NH 2 Molecular sieve. 0.45g Cu(saloph) dissolved in 60mL CHCl 3 In, add 1gMCM-41-NH 2 , the suspension was stirred at room temperature for 24 h, filtered, dried, extracted with methanol until the solution no longer changed color, and dried in vacuo to obtain Cu(saloph) / MCM-41.
[0025] Add mass ratio to the there-necked flask and be 2.16% Cu(saloph) / MCM-41, 0.51% triphenylphosphine, 8.65% benzene, 76.2% acetonitrile, 13.0% 30% H 2 o 2 , heated to 65°C, and refluxed for 6h under magnetic stirring. After the reaction was completed, it was cooled to room temperature, the catalyst was filter...
example 3
[0027]Based on 1g of SBA-15, the catalyst Cu(saldiamp) / SBA-15 was synthesized according to the following steps. 1g of SBA-15 molecular sieve was suspended in 15mL of anhydrous toluene, 5mL of 3-aminopropyltriethoxysilane was added, and nitrogen protection , stirred and refluxed for 10 h, filtered, washed with toluene and diethyl ether in turn, and dried in vacuum to obtain 1.3 g of SBA-15-NH 2 Molecular sieve. 0.38g Cu(saldiamp) dissolved in 60mL CHCl 3 In, add 1g SBA-15-NH 2 , the suspension was stirred at room temperature for 24 h, filtered, dried, extracted with methanol until the solution no longer changed color, and dried in vacuo to obtain Cu(saldiamp) / SBA-15.
[0028] Add mass ratio to the there-necked flask and be 2.16% Cu (saldiamp) / SBA-15, 0.51% triphenylphosphine, 8.65% benzene, 76.2% acetonitrile, 13.0% 30% H 2 o 2 , heated to 65°C, and refluxed for 6h under magnetic stirring. After the reaction was completed, it was cooled to room temperature, the catalyst wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com