A kind of environment-friendly metal carboxylate and preparation method thereof
A metal carboxylate, environmentally friendly technology, applied in the preparation of carboxylate, organic chemistry and other directions, can solve the problems of high production cost, low reaction temperature, lack of feasibility, etc., to achieve improved product quality, simple preparation process, processing The effect of easy-to-find equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
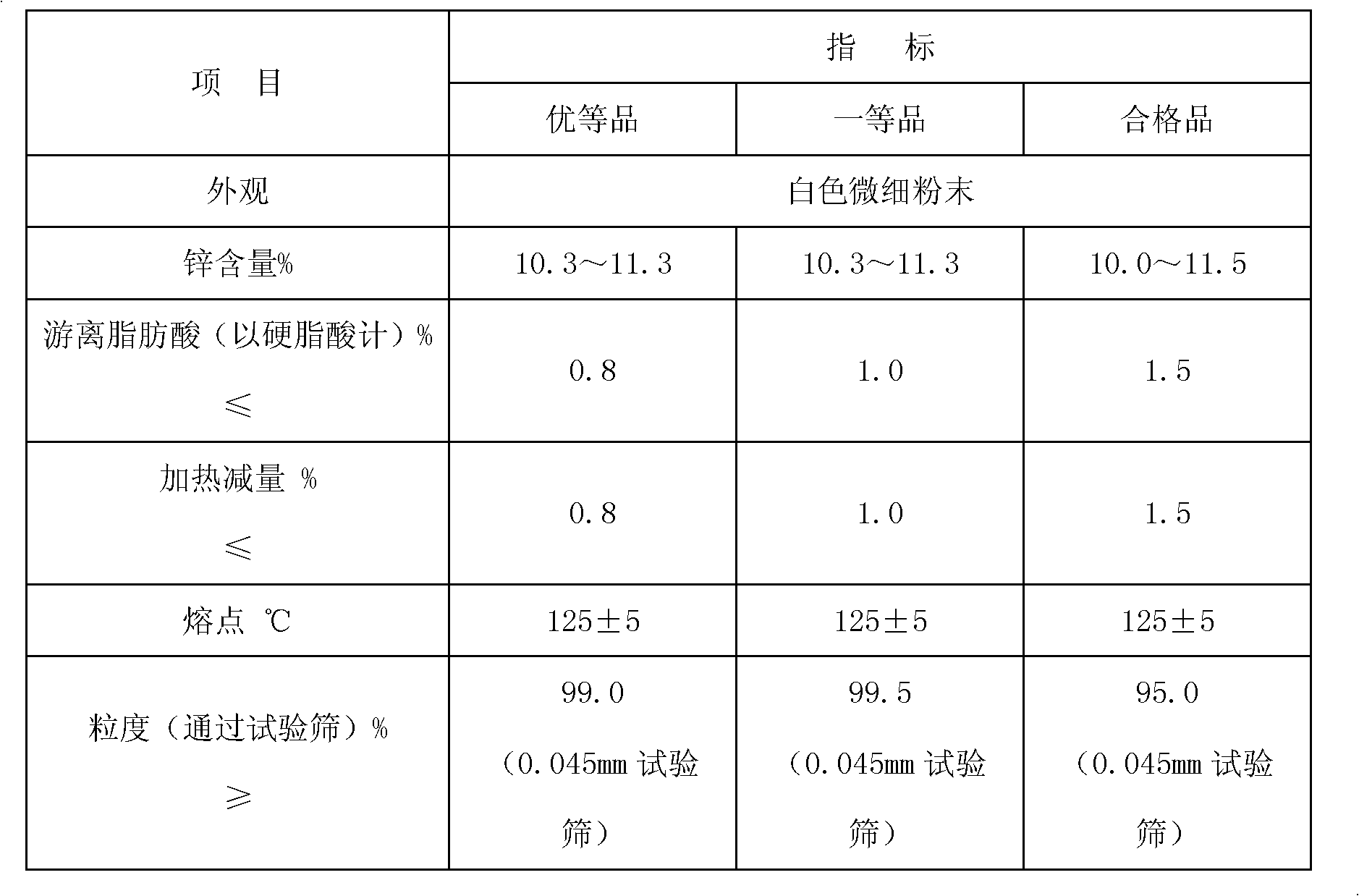
[0038] The preparation of embodiment 1 zinc stearate
[0039] 900kg of stearic acid (acid value 210) was heated and melted at 90°C, then heated to 135°C, added 135kg of zinc oxide (≥99.7%), mixed and dispersed uniformly, within half an hour, added 25kg of 30% The hydrogen peroxide catalyzed the reaction, and the temperature was kept for 1.5 hours. Then, it is cooled into solid flakes, and crushed by a high-speed collision pulverizer to obtain a zinc stearate product. Its quality indicators are: zinc content 10.8%; heating loss ≤0.8%; melting point ≥119°C; free acid ≤0.8%; fineness ≤75μm.
Embodiment 2
[0040] The preparation of embodiment 2 zinc stearate
[0041] 900kg of stearic acid (acid value 210) was heated and melted at 95°C, then heated to 135°C, added 146kg of zinc hydroxide (≥99.7%), mixed and dispersed uniformly, within half an hour, added 20kg 30 % hydrogen peroxide to catalyze the reaction, and keep the temperature for 1.5 hours. Then, it is cooled into solid flakes, and crushed by a high-speed collision pulverizer to obtain a zinc stearate product. Its quality indicators are: zinc content 10.6%; heating loss ≤0.8%; melting point ≥119°C; free acid ≤0.8%; fineness ≤75μm.
Embodiment 3
[0042] The preparation of embodiment 3 magnesium stearate
[0043]900kg of stearic acid (acid value 210) was heated and melted at 100°C, then heated to 138°C, and 101.8kg of magnesium hydroxide (≥96.0%) was added. After mixing and dispersing uniformly, 20kg was added in three times within half an hour. 30% hydrogen peroxide catalyzed the reaction and kept the temperature for 1.5 hours. Then it is cooled into a solid, and the product of magnesium stearate is obtained by pulverizing with a high-speed collision pulverizer. Its quality indicators are: magnesium content 4.8%; heating loss ≤1.5%; melting point ≥120°C; free acid ≤2.0%; fineness ≤75μm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com